Note: Descriptions are shown in the official language in which they were submitted.
CA 02210924 1997-07-21
PACLITAXEL ANALOGS, PREPARATION
AND USE AS ANTITUMOR AGENTS
Paclitaxel is a well known antitumor agent and has
been approved by the Food and Drug Administration for
5 treatment of ovarian and breast cancer. This drug is also
presently undergoing clinical trials for treatment of other
types of cancer. The world-wide supply of paclitaxel,
however, is limited to a finite number of yew trees and other
yew species containing relatively small amounts of paclitaxel
10 of which there is a serious shortage for human and animal
tumor treatment, and as well as for use in routine bioactivity
testing in the development of antitumor agents having
paclitaxel-like antitumor activity. Thus, alternate sources
of pa.~J.ita~~ce~. as well as <,ite:w,;~a.ce compounds having
15 pacl:~taxel~l~ke antitumor activity are highly desired.
Paclitaxel is most often present in combination with
its well known and structurally similar taxane,
cephalomannine. The structures of cephalomannine and
paclitaxel are shown below (I).
O
18
NH O
3. 7, 1,
O OH v
O~~
20
Paclitaxel
CA 02210924 1997-07-21
- 2 -
11 10 9
y 1. ~ O 12 16 5 (I)
x'15. 2 3
..
Cephalomannine
Paclitaxel and cephalomannine are natural products
5 found in the bark of the Pacific yew tree Taxus brevifolia,
and other yew species including T. baccata, T. cuspidata, T.
yunnanensis, T. chinensis, T. capitata, T. brownii and T. dark
green spreader. These compounds can also be found in
Cephalotaxus species such as Cephalotaxus mannii as well as
cultured plant cells and fungi.
Cephalomannine has been reported to be effective in
causing the remission of leukemic tumors. See U.S. Patent No.
4,206,221.
In accordance with this invention it has now been
unexpectedly discovered that certain novel paclitaxel analogs,
specifically 2",3" side-chain halogenated cephalomannines show
strong in vitro and in vivo paclitaxel-like efficacy in a
variety of tumors thereby providing a viable alternative to
paclitaxel and paclitaxel derivatives, such as Taxotere~.
20 The chemical structures of both cephalomannine and
paclitaxel contain eleven asymmetric carbon atoms, of which
nine are in the taxane ring and two are in the side chain at
carbon 13. Stereostructures of cephalomannine and paclitaxel
are shown below (II):
CA 02210924 1997-07-21
- 3 -
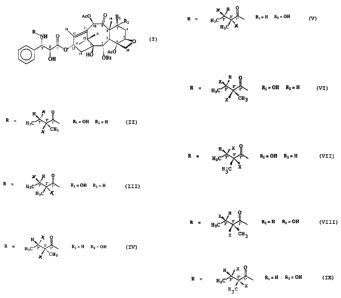
(II)
1. Paclitaxel R =
2. Cephalomannine; R =
The exocyclic 2",3" side-chain double bond in
cephalomannine along with the number of stereocenters present
in the structure of this compound suggests the possibility of
the existence of numerous stereoisomers of this taxane. For
example, cephalomannine can be distributed in two isomeric
forms wherein the hydroxyl group at carbon 13 is acylated with
phenylisoserine acylated in amino group by either (Z)- or (E)-
2-methyl-2-butenoic acid leading to (Z)- and (E)-cephaloman-
nines, respectively. In addition, it is known that cephalo-
mannine and paclitaxel can be epimerized at carbon 7 either
thermally, during chromatographic procedures or in acidic or
basic solutions to produce 7-epi-cephalomannine, which is
shown below (III). Miller, et al., J. Org. Chem., 40:1469
(1981); Chaudhary, et al., J. Org. Chem., 58:3978, (1993); and
blender, et al., CRC Press, Inc., Boca Raton, Fla. " (1995).
Thus, during halogenation the 2",3" side chain positions can
give rise to a mixture of diastereomeric products.
Therefore, in addition to that set forth above the
invention provides isolated and purified diastereomers of
2",3"-dihalocephalomannine and 2",3"dihalo-7-epi-
cephalomannine, which show strong antitumor efficacy.
Stereoview of Taxanes
I i~ i
CA 02210924 2002-09-06
- 4 -
Therefore, in addition to that set forth above the
invention provides isolated and purified diastereomers of 2",
3"-dihalocephalomannine and 2",3"dihalo-7-epi-cephalomannine,
which show strong antitumor effacy.
O
3. t8
O
4"
5 ' (III)
3, 2, 1\O~
0 off
7-epi-cephaloma.nnine
Brief Description of the Figures
Fig. 1 is a TLC separation of 2",3"-
dibromocephalomannine and 2",3"-dibromo-7-epi-cephalomannine
diastereomers (DiBr-I-IV).
Fig. 2 is an HPLC chromatogram of a mixture of
diastereomers DiBr-I, DiBr-II, DiBr-III and DiBr-IV.
Fig. 3 shows a comparison of the W spectra for
diastereomers DiBr-I, DiBr-II, DiBr-III and DiBr-IV.
Fig. 4 shows a comparison of the IR spectra for
diastereomers DiBr-I, DiBr-II, DiBr-III and DiBr-IV.
Fig.5 is an EI mass spectrum of diastereomer DiBr-I
which is the same fragmentation pattern for diastereomers
DiBr-II, DiBr-III and DiBr-IV.
Fig. 6 is a FAB' mass spectrum of diastereomer DiBr-
I which is the same spectrum for diastereomers DiBr-II, DiBr-
III and DiBr-IV.
Fig. 7 is the 1H-NMR spectrum of DiBr-I.
n
CA 02210924 2002-09-06
- 4a -
Fig. 8 is the 1H-NMR spectrum of DiBr-II.
Fig. 9 is the 1H-NMR spectrum of DiBr-III.
Fig. 10 is the 1H-NMR spectrum of DiBr-IV.
Fig. 11 shows the 13C-NMR spectra of DiBr-I, DiBr-
II, DiBr-III and DiBr-IV.
Fig. 22 are mean graphs of dose response of a
mixture of dibromo-cephalomannine diastereomers -I and -II in
a screen of sixty human tumor cell lines.
Fig. 13 are mean graphs of dose response of
paclitaxel in a screen of sixty human tumor cells.
Fig. 14 is a TLC separation of
dichlorocephalomannine (I and II) and (2"R.3"S) and
(2"S.3"R)-dichlorocephalomannine diastereomers (III and IV)
respectively.
Fig. 15 is HPLC chromotogram of a mixture of
respective dichlorocephalomannine diastereomers (I, II, III
and IV).
Fig. 16 is a comparison of UV spectra of pure
dichlorocephalomannine (I and II) and (2"R.3"S) and (2"S.3"R)
dichlorocephalomannine diastereomers (III and IV).
Fig. 17 is a comparison of IR spectra of pure
dichlorocephalomannine (I and II) and dichlorocephalomannine
diastereomers (III and IV).
Fig. 18 is a 1H-NMR spectrum of (2"R.3"S)
dichlorocephalomannine (I).
Fig. 19 is 1H-NMR spectrum of (2"S.3"R)
dichlorocephalomannine (II).
Fig. 20 is 1H-NMR spectrum of (2"R.3"R)
dichlorocephalomannine diastereomers (III).
Fig. 21 1H-NMR spectrum of (2"S.3"S)
dichlorocephalomannine diastereomers (IV).
Fig. 22 is a comparison of 13C-NMR spectra of
(2"R.3"S) and (2"S.3"R) dichlorocephalomannine (I and II) and
~i
CA 02210924 2002-09-06
- 4b -
(2"R.3"R) and (2"S.3"S)-dichlorocephalomannine (III and IV)
diastereomers respectively.
Fig. 23 is an EI mass spectrum of (2"S.3"S)-
dichlorocephalomannine diastereomer (IV) which is the same
fragmentation pattern for dichloro diasteromers (I), (II) and
(III) .
Fig. 24 is a FAB+ mass spectrum of (2"S.3"R)-
dichlorocephalomannine diastereomer (II) which is the same
spectrum for dichloro diastereomers (I), (III) and (IV).
Fig. 25 represents mean graphs of dose response of
(2"R.3"S) - dichlorocephalomannine diastereomer (I) obtained
from this invention in a screen of sixty human tumor cell
lines.
Fig. 26 represents mean graphs of dose response of
the (2"S.3"R)-dichlorocephalomannine diastereomer (II)
obtained from this invention in a screen of sixty human tumor
cell lines.
Detailed Description of the Invention
With Preferred F~nbodiments
The present invention provides novel anlogs of
paclitaxel, specifically isolated and purified 2", 3"
dihalocephalomannine and 2", 3" dihalo-7-epi-cephalommannine
diastereomers, which show strong in vitro and in vivo
paclitaxel-like antitumor activity in a variety of tumor cell
lines. The invention also provides methods for the
preparation of these compounds and their use in tumor
treatment.
CA 02210924 1997-07-21
- 5 -
In accordance with this invention, the
diastereomeric mixture of dihalocephalomannine analogs are
prepared in good yields from either relatively refined sources
of cephalomannine or from complex unpurified mixtures compris-
5 ing cephalomanine, paclitaxel and other taxane compounds. The
analogs are prepared by selective halogenation of the
unsaturated side-chain of the cephalomannine molecule, while
leaving other portions or moieties of the molecule or other
important taxane compounds in the mixture, such as paclitaxel,
intact.
Separation and purification of individual 2",3"-
dihalocephalomannine/dihalo-7-epi-cephalomannine diastereomers
from the mixture is accomplished by conventional methods, and
these compounds also show strong anti-tumor efficacy.
15 The selective halogenation is carried out by
reacting cephalomannine and/or 7-epi-cephalomannine under
conditions inclusive of a temperature and time effective to
selectively halogenate the 2",3" side-chain portion of these
compounds, and then separating the resulting less polar
20 mixture of dihalocephalomannine/dihalo-7-epi-cephalomannine
diastereomers from paclitaxel and other taxane compounds.
Individual diastereomers can be isolated from the mixture and
purified by standard chromatographic techniques and/or
recrystallization.
25 The synthetic methods of this invention are
advantageously independent of the concentration of
cephalomannine and 7-epi-cephalomannine present in various
complex or more refined mixtures of taxane compounds and can
utilize any source containing cephalomannine and/or 7-epi-
30 cephalomannine as starting material. Representative examples
of sources include the bark from various Taxus species, such
as Taxus brevifolia, Taxus baccata, Taxus yunnanensis, Taxus
chinenesis and Taxus wallichiana; from Cephalotaxus species
such as Cephalotaxus mannii, plant material; leaves, needles
35 and twigs from various Taxus and Cephalotaxus species,
extracts of biomass containing a complex mixture of taxane
type compounds, as well as in the downstream purification of
cephalomannine and 7-epi-cephalomannine produced from sources
CA 02210924 1997-07-21
- 6 -
such as cell cultures of Taxus and Cephalotaxus species and
cephalomannine-producing fungi.
In one example of this invention, a mixture of
taxanes comprising cephalomannine and/or 7-epi-cephalomannine
5 in addition to paclitaxel is treated with stoichiometric
quantities of halogen, such as for example, bromine or
chlorine dissolved in an inert solvent, preferably a
chlorinated solvent such as carbon tetrachloride, chloroform,
methylene chloride or ethylene dichloride. In a typical
10 treatment, for example, using a mixture containing
approximately 30 wt. % cephalomannine with halogen in carbon
tetrachloride results in a quantitative yield of a mixture of
2",3"-dihalocephalomannine diastereomers and the corresponding
2",3"-dihalo-7-epi-cephalomannine diastereomers. The general
15 reaction scheme (IV) is as follows:
a o
n +
~.- h
X z ~~.~e
1
~o 0
X
A r '+' I V
X I ~'y~l~y~
n
ow
l.Peddatd 3. 2'.3'-dihalocephalomannine
CA 02210924 1997-07-21
rw:v 1g ctl
,,~~ R2
18 11 g 8
R 1z 16
~NH O ~s 3 s
4
17 d''~~~. 2
3 2 1 O~ 13
14 Hd _ A~O
off asZ
wherein,
I. (2"R,3"S)-dihalocephalomannine
H X O
R - H3C 3- 2~ 1~ R1 =- OH RZ - H
X ...'CH3
II. (2"S,3"R)-dihalocephalomannine
H O
R - H3C 3- 2~ 1~ R1- OH RZ - H
H3C .,
III. (2"R,3"S)-dihalo-7-epi-cephalomannine
H ~( O
R - H C 3. 2° 1. R1 . H Rz - OH
3
x '~~-~H3
CA 02210924 1997-07-21
- 8 -
IV. (2"S,3"R)-dihalo-7-epi-cephalomannine
x H O
R1=H R,_=OH
R _ H3C 3~
HsC ..X
and X = halogen.
The resulting pure dihalo-diastereomers I-IV can be
separated and their chemical structures elucidated by
5 conventional analytical and physicochemical techniques.
Further in accordance with this invention, for
mixtures containing cephalomannine and/or 7-epi-cephalomannine
and from about 0.01% wt. to about 95.05% wt. paclitaxel, the
process is similar to that described above. The mixture is
10 first dissolved in an inert solvent, preferably carbon
tetrachloride, chloroform, 1,2-dichloroethane or methylene
chloride which is reacted with a halogen, for example, a
solution of bromine or chlorine in an inert chlorinated
solvent, and the reaction stirred until cephalomannine is
15 completely reacted. It is preferred that the reaction be run
at temperatures between -20°C and 20°C and more preferably
between -5°C and 5°C, preferably in the dark. The preferred
halogen solution is bromine or chlorine in carbon
tetrachloride of from O.O1M to O.1M. To ensure that reaction
20 conditions favor the production of the desired 2",3"-
dihalocephalomannine and/or 2",3"-dihalo-7-epi-cephalomannine
diasteromeric reaction products, reaction progress can be
conveniently monitored by conventional analytical techniques,
for example, HPLC, and the appropriate reaction conditions
25 maintained.
The reaction mixture containing taxane impurities
can then be separated and purified by conventional methods
such as chromotography and recrystallization and the
individual separated and purified diastereomers made available
30 for antitumor treatment.
CA 02210924 1997-07-21
_ g _
Conventional wisdom would lead one to expect that
the use of halogen in the presence of taxane compounds having
several functional groups would result in undesired side
reactions, thereby depleting the concentration of
5 cephalomannine and/or 7-epi-cephalomannine and halogen without
generating the desired dihalocephalomannines, or appreciable
yields thereof. It would also be expected that other valuable
taxanes such as paclitaxel would be degraded by such halo-
genation. However, in accordance with this invention it has
10 been found that selectivity for halogenation of the 2",3"-
side-chain double bond in cephalomannine and 7-epi-cephaloman-
nine is very high under controlled conditions, with paclitaxel
neither significantly degraded nor halogenated. As mentioned
above, any undesired degradation or reaction products during
15 halogenation can be avoided and the effective conditions
adjusted appropriately without undue experimentation by
monitoring the reaction, for example, by HPLC.
The molar equivalents of halogen used in this
invention are dependent upon cephalomannine and/or 7-epi-
20 cephalomannine content and presence or absence of other
unsaturated compounds. In general, a less pure mixture, i.e.
a mixture containing large amounts of unsaturated taxanes
relative to cephalomannine and 7-epi--cephalomannine will
require a higher molar equivalent of halogen to halogenate all
25 or substantially all of the cephalomannine and/or 7-epi-
cephalomannine present in the mixture. Structures of various
other unsaturated taxanes typically present along with
cephalomannine, 7-epi-cephalomannine and paclitaxel in plant
extracts are shown below (V):
CA 02210924 1997-07-21
- 10 -
,ro 0 0
n y ~ ,v n w
L pscGtnd X i aprvodaiw
1
(V)
~.0 0 0
n
X- I
n n
a~ °"' oa aon
ow
l.pedita:d 3. 2',3'-dihalocephalomannine
The following examples are provided to illustrate preferred
embodiments of the invention, specifically selective
bromination and chlorination of samples containing
5 cephalomannine, 7-epi-cephalomannine, paclitaxel and other
taxanes, all present in varying amounts, and without
significant undesirable reactions and/or degradation, for
example, of paclitaxel. Examples are also provided which
demonstrate the antitumor efficacy of the inventive
10 dihalocephalomannine/dihalo-7-epi-cephalomannine compounds.
These examples are only intended for the
illustration of some preferred embodiments of this invention,
and are not intended to limit the scope of the invention as
defined by the claims.
15 EXAMPLE 1
BROMINATION OF A PARTIALLY PURIFIED
MIXTURE CONTAINING CEPHALOMANNINE
A solution of 0.63g of 91.5% cephalomannine (0.0007
moles), also containing about 6-7% paclitaxel, dissolved in
20 150 ml carbon tetrachloride was added to a 500m1 three neck
round bottom flask fitted with a 250 ml separatory funnel.
CA 02210924 2002-09-06
- 11 -
EXAMPLE 1
BROMINATION OF A PARTIALLY PURIFIED
MIXTURE CONTAINING CEPHALOMANNINE
A solution of 0.63g of 91.5% cephalomannine (0.0007
moles), also containing about 6-7% paclitaxel, dissolved in
150 ml carbon tetrachloride was added to a 500m1 three neck
round bottom flask fitted with a 250 ml separatory funnel.
The flask was then immersed in an ice-salt bath. When the
temperature reached -5°C, a solution of bromine (0.1221 g) in
carbon tetrachloride (76.31 ml, 0.01 M) was added slowly with
stirring at such a rate that the reaction temperature did not,
exceed 5°C. The cephalornannine to bromine ratio was 1:1.1
mole. This addition required about three hours and the
resulting solution was light brown and cloudy.
The bromination was monitored by HPLC analysis every
hour. The reaction was completed when all the cephalomannine
present was converted to the 2", 3"-dibromoderivative, which,
based on HPLC analysis, required approximately a hrs. The
reaction mixture was light yellow to colorless, due to the
consumption of the bromine, in contrast to the darker starting
solution.
The reaction mixture was then transferred to a one
litre separatory funnel and first washed with 0.5% aqueous
sodium sulfite (300 ml), 0.5% aqueous sodium bicarbonate (300
ml) and then twice with deionized water (200 ml each) to a
final pH 6.5. The combined aqueous layer was extracted once
with CH2C12 and the CHzCl2 layer mixed with the previous organic
extract. The organic layer was next dried over NaZSO"
filtered, and evaporated to dryness. The yield was 0.76 g of
a light cream-colored solid which is approximately a 100%
yield based on the starting material.
The cream colored solid material was chromatographed
on a column of silica gel (50g, ICN Silitech, 32-63 D, 60 A)
using the solvent mixture acetone:CHZC12 (10:90) as the eluent.
Fifty ml fractions were collected and. checked by TLC (Silica
gel 60 Fzs4°, Merck #5554, developed with acetone/CH2Clz . 20/80,
detected using vanillin-sulfuric acid in methanol spray
CA 02210924 1997-07-21
- 12 -
reagent). The fractions with a single spot at Rf = 0.64
(fractions #26 - #38) were mixed, concentrated to dryness to
yield 0.485 g of a light cream powder, which was recrystal-
lized to white crystalline solid, mp 158'C, and identified as
5 2", 3"-dibromocephalomannine by physico-chemical methods (TLC,
HPLC, UV, IR, NMR, MS). The yield was estimated to be 70% on
the basis of starting cephalomannine.
EXAMPLE 2
BROMINATION OF A CRUDE MIXTURE CONTAINING
CEPHALOMANNINE, PACLITAXEL AND
OTHER TAXANE-TYPE COMPOUNDS
Using similar apparatus as used in Example 1, a
sample of crude paclitaxel (2.0 g) having a mixture of 51.2%
paclitaxel 28.8% cephalomannine, and about 20% other taxanes
15 or non-taxane impurities based on HPLC was dissolved in 150 ml
carbon tetrachloride and 150 ml CHzCl2, to yield a clear, light
yellow solution. The flask was immersed in an ice-salt bath
and stirred. When the temperature reached -5°C, a solution of
0.1332 g 100% bromine in 83.13 ml (0.01 M) of carbon
20 tetrachloride (1 mole cephalomannine . 1.2 moles bromine) was
added to the solution at such a rate that the temperature of
the reaction mixture did not exceed 5°C. The addition required
about three hours and resulted in a cloudy, brownish-yellow
solution. After the addition of bromine was completed, the
25 reaction was allowed to continue under the same conditions for
an additional 8 hours, with HPLC analysis of the paclitaxel
and cephalomannine performed every hour. The reaction was
complete when the solution is colorless or light yellow and
all the cephalomannine has been converted to the dibromo
30 derivative. If after the additional 8 hours the solution
still contained more than 1 - 2% cephalomannine, keeping the
initial conditions, 10 ml 0.01 M bromine in carbon
tetrachloride was added dropwise and allowed to react for 1
hour before analyzing again with HPLC.
35 Excess bromine from the reaction mixture was removed
by washing with 0.5% aqueous NaZS03 (300 ml), 0.5% aqueous
NaHC03 (200 ml), and deionized water (2x200 ml). The reaction
~I i
CA 02210924 2002-09-06
- 13 -
mixture was dried using anhydrous NazSO, and concentrated to
dryness under high vacuum to yield 2.35 g of dry light cream
to white powder. The dry material was then purified on a
silica gel column under the conditions listed in Example 1.
The ratio between the mixture to be separated and the silica
gel was 1: 60, thus 120 g silica gel were used. Each fraction
was checked by TLC and every third fraction by HPLC. Frac-
tions with the same Rf in TLC and same retention time in HPLC
were mixed to afford two combined fractions. Fractions (#25-
#39) which showed a single TLC spot with Rf 0.64 represented
dibromocephalomannine and fractions (#41 - #81) which showed a
single TLC spot with Rf 0.49 represented paclitaxel.
Fractions #25 - #39, after concentration to dryness
at about 40°C under high vacuum, yielded a white to light
15. yellow solid, 0.460 g, (66.6% theoretical yield) with a m.p.
158' - 160°C (chromatographic purity 96.19%) as determined by
TLC.
TLC materials were employed as follows: R~ = 0.64
(single spot) on Silica gel 60 Fz54° Plate (Merck #5554)
Solvent system: acetone . CHZCIz (20:80)
Spray Reagent: vanilin/sulfuric Acid in methanol
Mass Spectrum [FAB]' of the obtained
dibromocephalomannine:
[M + H]' - 990, 992, 994
[M + Na]' - 1014
[M + K] ' - 1030
Concentration of the second combined fractions (#41
- #81) yielded 1.16 g ()100% theoretical yield) paclitaxel,
which was recrystallized using 50 . 50 acetone/hexane,
filtered, washed with the same ratio of cooled solvent and
dried under high vacuum at 40°C for 24 hrs. The yield was
0.902 g (45.11% based on the starine material and 88.08% based
on the HPLC analysis of paclitaxel in the starting material)
of a white crystalline material with a m.p. of 214°C - 216°C.
I ' ~I
CA 02210924 2002-09-06
- 14 -
TLC analysis materials: Rf = 0.49 in the prese.~.ce of
authentic sample on silica gel 60 Fzsa" Plate [Merck #5554)
Solvent system: acetone/CH2C12 (20:80)
Spray Reagent: vanilin/sulfuric acid in methanol
Both the W and the IR spectra of the resulting
material match those of pure paclitaxel thereby demonstrating
the high selectivity of the bromination reaction for the 2",
3" unsaturated side chain positions of cephalomannine while
leaving its close analog paclitaxel untouched.
EXAMPLE 3
SCALED-UP EXAMPLE ILLUSTRATING BROMINATION
OF A CRUDE MIXTORE CONTAINING CEPHALOMANNINE
A solution of 10.00 g crude paclitaxel (on the basis
of HPLC analysis the content was 28.8% cephalomannine, 51.2%
paclitaxel and approximately 20% other taxane or non-taxane
impurities) was dissolved in 1.5 1 carbon tetrachloride in a
2 1 three-necked flask fitted with a S00 ml separatory funnel,
reflux condenser, thermometer and magnetic stirrer and
immersed in an ice-salt bath. The reaction mixture was
stirred until the temperature reached -5°C and then 41.2 ml of
0.1 M bromine (0.665g bromine) in carbon tetrachloride was
added dropwise for about 3 hours. The molar ratio between
cephalomannine and bromine was 1 . 1.2. The temperature did
not exceed 5°C. After the bromine addition was completed,
stirring was continued while maintaining the temperature at
-1°C to 5°C. The reaction was monitored by HPLC every hour
until all the cephalomannine had been converted to the dibromo
derivatives (approximately 8 hrs.). The final color of the
1500 - 1600 ml of solution was light yellow or cream,
depending on the color of the starting mixture and the
possible presence of a small excess of bromine.
To remove any trace of bromine, the reaction mixture
was washed with 0.5% aqueous Na2S03 (500 ml), 0.5% aqueous
NaHC03 (500 ml), and deionized water (2x500 ml). The reaction
mixture was next dried with anhydrous Na2S0, and concentrated
i
CA 02210924 2002-09-06
- 15 -
to dryness under vacuum to yield 13.20 g of a light cream to
white solid material.
This material was chromatographically separated on a
silica gel column under the conditions listed above in
Examples 1 and 2. A 100 x 5 cm glass column was prepared by
the slurry method with 600 g silica gel (ratio 1:50). The
column was eluted with acetone/CHZC12 (10 . 90). One 1 of
acetone/CH2C12 (25 . 75) was used as a final column wash.
Every fraction was analyzed by TLC and every third fraction by
HPLC. Fractions #11 - #22 had a single spot at Rf = 0.64 and
their combination, concentration and drying (40°C, high
vacuum), yielded 3.25 g (95%) of 2",3"-dibromocephalomannine
as a white to light yellow solid.
Analysis of this compound is as follows:
m.p.. 158 - 160°C.
Rf = 0.64 (single spot) on silica gel 60 F2sa
plate [Merck #5554].
Solvent system: Acetone/CH2C12 (20 . 80)
Spray Reagent: Vanilin/Sulfuric Acid in
Methanol.
Elemental Composition and Molecular Weight (on the
basis of HR FAB')
C45H54N~1479Br2 [M + H] ' .
Calculated: 990.191000
Found: 990.191103 (nm = 0.1 ppm)
CASH54NO1479Br81Br [M + H] ' .
Calculated: 992.181000
Found: 992.189057 (nm = 8.1 ppm)
C45H54N~1481Br2 [M + H] ' .
Calculated: 994.175000
Found: 994.187011 (om = 12.1 ppm)
CasHs3N~iaNa'9Br81Bz' [M + Na] ' .
Calculated: 1014.161000
Found: 1014.171002 (nm = 9.9 ppm)
CA 02210924 1997-07-21
- 16 -
CasHs3N~i4K~9BrelBr [M + K] ' .
Calculated: 1030.097000
Found: 1030.144940 (nm = 46.5 ppm)
[oc] DZS-_40.207' (c 0 .29, MeOH)
5 UV Spectrum in CH30H [Amax nm, ( a ) ] : 274 . 2 ( 1550 . 8 ) ; 227 . 1
(18610.4); 221.8 (18325.1)
IR Spectrum in KBr (cm-1) 3500, 1105, 1070 (tert & sec OH)
3420, 1670, 1580 (-CONH-)
3110, 3060, 1605, 1505, 770, 710
(monosubt. aromatic cpds.)
3060, 2960, 2915, 2870, 1465,
1370
(-CH3, -CH2-, =CH-)
3020, 1670, 1310, 980 (double
bond)
1730, 1270 (aromatic esters)
1715, 1240 (>C=O)
1730, 1180 (acetates)
855 (epoxy rings)
520 (bromo compounds)
1H sNMR in CDC13 (300 MHz) : 1 . 94 (d, 3H, -COC (Br) CH3 -5" )
(ppm;side chain protons only) 1.98 (d,3H, -HC(Br)CH3 -4")
4.63 (qt, 1H, >CH(Br) -3")
13C NMR (300 MHz) 170.21 and 170.25 (C-1')
(in ppm; side-chain C only)
72.76 and 72.90 (C-2')
172.26 and 172.32 (C-1")
54.34 and 54.52 (C-3')
69.71 and 69.88 (C-2")
30 55.13 and 55.35 (C-3")
30.39 and 30.77 (C-4")
27.21 and 27.62 (C-5")
CA 02210924 1997-07-21
- 17 -
EI-MS 568,551,509,491,449,431,405,391,386,329,
(m/z)
(the main fragments)
326,308,278,264,245,217,200,188,159,149,
122,105,91,83,77,55,43.
DCI-MS(m/z)
(the main fragments)
569,552,510,492,474,450,432,
424,392,387,370,329,327,309,279,265
10 264,246,218,200,188,167,149,125,124,
106,101,100,91,83,69.
FAB' - MS: 1030 (M + K]'; 1014 [M + Na]'; 992
(positive ion mode) (M + H]'(See Elem. Anal.); 974 (M-H20]';
(m/z) 932 [M-AcOH]'; 914 (M-AcOH-H20]'; 912
15 [M-HBr]'; 870 (M- BzOH]'; 854 (870-
HZO-2H] ; 832 (M2-HBr]'; 705 [M-243-
Ac] '; 569 (T]'; 551 (T-H20] ; 509 (T-
AcOH]'; 491 [T-AcOH-H20]'; 448 [T-
BzOH] '; 429; 424 (SHZ] '; 413 ; 405 [S-
20 H20] '; 391 [S-0-H20] ';
387 [T-AcOH-BzOH]'; 376; 347 [S-0-
CO-HCHO]'; 338:327 [387-T-AcOH]';
315; 284 [327-Ac]', 279; 264 [832-T] ' or
[424-2HBr]'; 246 [264-Hz0]'; 231; 218
25 [264-HCOOH]';188; 167[S-CSHe0NBr2]';
149 (167-H20]'; 133; 122 [BzOH]';
113:105 [Bz]'; 91 [C,H,]'; 83; 77 [C6H6]';
76; 57; 55;
(T=taxane ring in the compound; S-
30 acid (side) chain in the compound.)
HPLC:
Condition 1: Column CN 10~,(250x4.6mmn)
Solvent System CH3CN:H20 (40:60)
Flow Rate 1 mL/min
35 Detector Waters 490uv at 227nm
Injection volume 20~,L
RTz n , 3 ~ -dibromocephalomannine 2 6 . 0 6 min .
~i i~
CA 02210924 2002-09-06
- 18 -
Condition 2: Column Curosil G~ 6~ (250x3.2mm)
Solvent System CH,CN:HzO (45:55)
Flow Rate 0.75 mL/min
Detector Waters 490uv at 227 nm
Injection Volume 20 ~.L
RT2,,,.,_dibromocephalomannine 2 diastereomeric forms:
RTI = 23.53
RTII = 24.50
Thermogravimetric Analysis (TGA): 28°C (100.0%),100°C
(99.64%),
(Temp. and % decomposition) 150°C (98.88%),175°C,
(95.35%) , 180°C
(86.74%),200°C
(60.38%) , 250°C (45 . 03%) .
Differential Scanning Calorimetry (DSC): 173.76°C,
187.73°C.
As demonstrated from the following analysis,
bromination of the crude paclitaxel mixture shows surprisingly
high selectivity for the 2", 3~~, positions of the unsaturated
side chain of cephalomannine, while leaving paclitaxel
untouched.
The fractions from #26 to #68 which had a single
spot in TLC (Rf 0.49, the same as the authentic sample of
paclitaxel) and a single peak in the HPLC, were combined,
concentrated and dried, (40°C, high vacuum lmm to 2mm) to yield
6.10 g of a white solid. This material was crystallized from
60 ml of a mixture of acetone/hexane mixture (50:50),
filtered, washed with the same ratio of cooled solvents and
dried under high vacuum at 40°C (24 hrs.) to obtain 4.84 g
(92%) of a white crystalline solid identified by comparison to
an authentic sample as paclitaxel.
i ~i
CA 02210924 2002-09-06
- 19 -
Analysis is as follows:
m.p.. 214 - 216°C
Rf:0.49 (in the presence of the authentic sample)
Silica gel 60 FzSa~ plate (Merck #5554
Solvent system: acetone/CH2Clz (20:80)
Spary Reagent: Vanillin/Sulfuric Acid in
Methanol
Elemental Analysis:
C4,H51014N : %C %H %N
Calculated 66.11 6.02 1.64
Found 65.97 5.89 1.63
[«]D~5=-51.104°(c 0.33, MeOH)
W Spectrum in CH30H:
(Amax in nm, (e) 227.2 (29824.1)
208.0 26256.3)
IR Spectrum (KBr)(cm-1) 3500, 1105, 1070 (tert. & sec. OH)
3430, 1650, 1580 (-CONH-)
1610, 1520, 780, 710 (monosub.
aromatic rings) 2950, 2910,
1480, 1450, 1370
(-CH3, -CHZ-, >CH-groups) 3020, 1315,
980(double bond)1725, 1270
(aromatic esters)1710, 1240 (>C=O)
850 (epoxy rings)
1H NMR Spectrum : 1.88 (S,lOH,C-1); 5.66 (d,lH, C-2);
(300 MHz; CDC13) 3.82 (dd,lH,C-3); 2.38 (S,3H, CH3C00
(ppm) at C-4); 4.94 (dd,lH,C-5); 1.88
(ddd,lH,C-6); 2.48(ddd,lH,C-6);
2.53(d,lOH,C-7); 4.38 (dd,lH,C-7);
6.27 (S,1H,C-10); 2.23 (S,3H,CH3C00
at C-10); 6.20 (qt,lH,C-13); 2.27
(ddd,lH,C-14); 2.33 (dd,lH,C-14);
1.13(S,3H,C-19); 1.23 (5,3H,
C-18);1.78(S,3H,C-18); 1.68(S,3H,
C-19);4.20(dd,lH,C-20); 4.30(5,1H,
CA 02210924 1997-07-21
- 20 -
C-20);3.77(S,1H,C-2'); 4.78(ddd,lH,
C-2'),5.20(ddd,lH,C-3'),7.10(d,lH,N-1);
7.30-7.53(m,lOH,p-&m-protons at
aromatic rings Al, B1, & C1) ;
5 7.64(t,lH,A1-p);7.72(dd,2H,
C1-o) ; 8 . 11 (dd, 2H,A1-o) .
13C NMR Spectrum 79.1(C-1);75.1(C-2);45.8(C-3);81.2
(300 MHz, CDC13) (C-4);84.4(C-5); 35.6(C-6);72.1
(ppm) (C-7);56.7(C-8);203.6(C-9);75.6(C-
10);
133.3(C-11);141.9(C-12);72.3
(C-13);35.7(C-14);43.2(C-15);
21.8(C-16);26.9(C-17);14.7(C-18);
9.5(C-19); 76.5(C-20); 73.3
15 (C-2');55.1(C-3');20.7(CH3C0) at
C-10; 22.6(CH3C0 at C-4);170.3(CH3C0
at C-10); 171.1(CH3C0 at C-4);167.0
(ArCO-Al) ; 167. 0 (ArCO-C1) ;
172.7(PhISCO-);129.3(aC-Al);133.8
(aC-B1) ; 138. 1 (aC-C1) ; 130 .3
(o-C,Al) ; 127.0 (o-C,B1) ; 127. 0
(o-C, C1) ; 128 .7 (m-C,Al) ; 128 . 6
(m-C, B1) ; 129 . 0 (m-C, C1) ; 133 . 6 (p-C, Al) ;
131 . 9 (p-C, B1) ; 128 . 3 (p-C, C1) .
25 EIMS : [M} '=853 568 [T] '; 550 [T-H20] '; 508 [T-AcOH] '; 490
(m/z, the main [T-AcOH-H20] '; 448 (T-2AcOH]' or
fragments) [T-BzOH]';386[T-AcOH-BzOH]';
326 (T-BzOH-2AcOHJ '; 308 [326-Hz0]'; 286 (M-T] '
or [S]';280;268 [S-0]';240 [S-O-CO]';
30 210 [S-O-CO-HCOH] '; 122 [BzOH] ';
105 [8z]'; 91 [C.,H,]';
77 [C6H5] '; 51; 43 [Ac]'.
i
CA 02210924 2002-09-06
- 21 -
DC/MS:(M + H]'=854 569;551;509;492;449;387;327;
(m/z; the main 311;287;269;240;224;222;210;165;
fragments) 149;123;105;92;71.
FAB MS:(positive ion mode):
(m/z; the main 892 [M+K]'; 876 [M+Na)'; 854 [M+H]'; 569; 551; 523 ;
fragments) 509;495;369;327;286;240;210;177;155;149;
119;105;85;69.
FAB MS:(negative ion mode):
852 - (M - H]
HPLC:
Column: ~Bondapak" Phenyl
Solvent System: CH3CN:CH,OH:H20-132:20:48
Flow Rate: 1mL/min
Detector: Waters 490uv at 227 nm
Injection volume: 20~,L
TGA: 50°C (100.0%),205oC (99.86%),215'C (99.10%),220°C
(92.19%), 250°C (56.66%),275°C (45.92%).
DSC: 210°C.
Water content (%H20): 0.90%(Karl Fischer)
CA 02210924 1997-07-21
- 22 -
EXAMPLE 4
ISOLATION AND PURIFICATION OF
2",3"-DIBROMOCEPHALOMANNINE DIASTEREOMERS
4.1 Raw materials
5 Batches of crude plant extracts from Taxus
yunnanensis having approximately 15-40% cephalomannine, 50-70
% paclitaxel, and approximately 20-35 % other taxane/non-
taxane components were obtained either from Seattle, Oregon,
Western yew (T. brevifolia), or from the Peoples Republic of
10 China (T. yunnanensis or T. wallachiana). Bromine reagent was
obtained from Fisher Scientific. Silica gel used was ICN
Silitech, 32-63 um, 60 P., ICN Biomedicals, Inc., Aurora, OH.
All solvents used were either HPLC or ACS grade and were
obtained from Spectrum Chemical Mfg. Corp. Purified water
15 used was deionized in-house.
4.2 Bromination of Crude Plant Extract
Crude plant extract (10.0 g, 26.4 % cephalomannine)
was dissolved in chloroform so that a total of 250 ml solution
was obtained. To the solution cooled in an ice bath and
20 continually stirred with a magnetic stirrer was added carbon
tetrachloride (4750 ml). To the cooled solution (4°C) was
added dropwi.se 0.1 M bromine in carbon tetrachloride (40 ml).
HPLC analysis of this mixture indicated a ratio of paclitaxel
to cephalomannine peak areas 2.6 to 1. The reaction mixture
25 was stirred in the dark with the temperature gradually rising
to 15°C. After 7 hrs of reaction, an additional 7 ml 0.1 M
bromine in carbon tetrachloride was added and the reaction
continued at 15°C. After an additional 8 hrs of reaction, the
final portion of 7 ml O.1 M bromine in carbon tetrachloride
30 was added and the reaction continued at 15'C overnight (14
hrs). Subsequent HPLC analysis of the mixture showed a ratio
of paclitaxel to cephalomannine peak areas 11 to 1. This ratio
increased to 12.3 to 1 after another 7 hrs of reaction. The
mixture was then washed with 5000 ml 0.2% aqueous sodium
i i i ~ I
CA 02210924 2002-09-06
- 23 -
sulfite solution. The pH of the aqueous layer was 8Ø This
was followed by two washes with water (2x5 1).
The pH of the first and second water washes were 6.5
- 7.0 and 6.0 - 6.5 respectively. The combined aqueous layer
was reextracted with 5 1 chloroform. The organic layers were
combined, dried with anhydrous sodium sulfate (500 g>, and
evaporated to dryness using a rotary vacuum evaporator at 40'C.
The solid residue (13.64 g) was purified by chromatography.
4.3 Chromatographic Purification
of Brominated Material
The thus obtained brominated material (13.64 g) was '
purified by medium pressure chromatography using a column (6.9
cm i.d., 70 cm long) packed with silica gel (ICN Silitech, 32-
63 um, 60 A) by the slurry method using 1.5% methanol in 1,2-
dichloroethane. The sample dissolved in the same solvent was
loaded and eluted at the rate of 50 ml/min. Total 55
fractions (500 ml each) were collected. The fractions were
analyzed by TLC, with the TLC plates developed with 10%
methanol in 1,2-dichloroethane and detected with I% vanillin
in 50/50 sulfuric acid-methanol. Dibromo-7-epi-
cephalomanrines eluted in fractions 10-14 and yielded 1.42 g
solids following evaporation of solvents. Likewise, the
dibromocephalomannines eluted in fractions 24-28 and yielded
1.64 g solids following evaporation of solvent. Individual
diastereomers of dibromocephalomannine and the corresponding
7-epi-cephalomannine were subsequently separated and isolated
by semi-preparative HPLC, discussed below in 4.4.
Evaporation of medium pressure chromatographic
fractions 34-54 yielded 4.79 g pure paclitaxel, m.p. 214 -
216°C, with analytical data determined by L1V, IR, HPLC, MS.
NMR, which is the same as presented in C1.S. Patent No.
5,840,748.
CA 02210924 2002-09-06
- 24 -
4.4 Separation of 2°, 3" -Dibromocephalomannine
and 2", 3" -Dibromo-7-epi-cephalomannine Diastereomers
The final purification of dibromocephalomannine and
dibromo-7-epi-cephalomannine diastereomers from other
impurities was accomplished bf semi-preparative HPLC (WatErs
Deltaprep" 3000) using 3 waters Deltapak C18 column, 100A,
mcr. x 30 cm with 50% acetonitrile in water as the mobile phase
at a flaw rate of 15 ml/min. Peak elution was monitored using
a waters Lambda Max Model 481 W detector set at 227 nm.
l0 Portions of 200 mg of material dissolved in methanol (2 m1>
were injected into the column. Elution of dibromocephaloman-
nine diastereomer I peaked approximately at 54 min, and
diastereomer II at 56 min. Likewise, the dibromo-7-epi-
cephalomannine diastereomer III peaked at approximately 104
min. and the corresponding diastereomer IV peaked at 112 min.
respectively. Fractions collected from repeated injections
were pooled and evaporated at 40'C under reduced pressure to
remove the organic solvent. The crystallized solids were
filtered, washed with water, and dried in a vacuum oven at 40'C
2o to yield pure dibromocephalomannine and dibromo-7-epi-
cephalomannine diastereomers. The preparation, separation and
structures of the obtained diastereomeric dibromo compounds,
(I) (2"R, 3"S)-dibromocephalomannine, (DiBr-I)
(2"R, 3"R)-dibromocephalomannine;
(II) (2"S, 3"R)-dibromocephalomannine, (DiBr-II)
(2"S, 3"S)-dibromocephalomannine;
(IhI) (2"R, 3"S)-dibromo-7-epi-cephalomannine, (DiBr-III)
(2"R, 3"R)-dibromo-7-epi-cephalomannine;
(IV) (2"S, 3"R)-dibromo-7-epi-cephalomannine, (DiBr-IV)
(2"S, 3"S)-dibromo-7-epi-cephalomannine are shown in
scheme VI:
~i
CA 02210924 2002-09-06
_7C,~
S=heme VI:
wro o ~.o A.o o
,. - "
r ,, O ~ r~( I r r ~ ~i r r n "
., " , ° . . o
1 . ' r 1 " . ' r r r
n n , . PH " , 1 r 1W ~ ,
,,~ HC~~ As0 " H° Ae0 M0
° ~ N o
m r~ ° r~
Prditaxe! Cephabnuaaiae 7 - epi - crphdomaaninc
B rZ
(CCW
(CHCIy)
(C~il~
(CiE~~3
s1o o ,"p
" " ~ ~ , r r " . " ~~ r° "
1 r r " 1 r , 1
11 1 ~ p , ,
1 1 ~
1
r r r " r ~ " -~ /~ r r ~ ' " .t. of , 1
.
, ~ ,. H A.o , H, A.o "
o~ i(
° n
Paditsxel 3", 3" - dlbrvmn - ceplalomudas Z", 7" - Nbr~ ~ 7 -,eoi -
aplaleamalae
Separation
Analogue I x2"R , 3"S) - dibromocephalomaonine AnalogneI I(2"S , 3"R) -
dibromocephalomaanine
- (2"R,3"R)-dibromocephalomannine - (2"S,3"S)-dibromocephalomannine
Paclitaxel ~
Analogue~$(3"fit , 3"S) - dlbromo-?-epi-cephalomanoine Malogu~.~2"S , 3"R) -
dibromo-~.epi~ephalomannine
- (Z"R,3"R)-dibroroo-7-epi-cephalomannine - (2"S,3"S)-dibromo-7-epi-
cephalomannine
Paclitaael Analogues {Brominated)
~i i i~ i
CA 02210924 2002-09-06
- 26 -
Analytical characterization of the diastereomers is
as follows:
FIG. 1 is a TLC separation of 2",3"-
dibromocephalomannine and 2",3"-dibromo-7-epi-cephalomannine
diastereomers (DiBr-I-IV) as summarized below in Table 1.
TABLE 1
lane Coae~ouad
(1) DiBr-I
(2) DiBr-II
(3) DiBr-III
(4) DiBr-IV
(P) pac-litaxel
plate: silica gel 60 Fzsa' (Merck #5554)
solvent system: a) 10% CH,OH in 1,2-dichloroethane
b) hexane/chloroform/EtOAc/CH30H
20/60/15/5
reagent: a) W light
b) vanilin/HZSO, in methanol
FIG. 2 is an HPLC chromatogram of a mixture of
diastereomers (I) DiBr-I; (II)DiBr-II; (III)DiBr-III; and
(IV)DiBr-IV. Equipment and conditions employed in generating
this chromatogram are the following:
Column: ES Industries FSP (pentafluorophenyl)
4.6 mm ID x250 mm, 5 um particle size,
60 A pore size
Solvent System: water/acetonitrile/methano1,41:39:20
Flow Rate: 0.50 ml/min., isocratic
Detector: Waters 990T" photodiode array detector,
monitored at 227 nm
Injection Volume: 20u1
FIG. 3 shows a comparison of the W spectra for
diastereomers DiBr-I, DiBr-II; DiBr-III and DiBr-IV in CH30H.
The spectra are summarized below in Table 2.
i I 1
CA 02210924 2002-09-06
- 27
TAB 2
I ,R ~ max(nm)
Di8r-I 226.0 14732
DiBr-II 226.0 12415
DiBr-III 219.4 37900
DiBr-IV 218.4 20013
FIG. 4 shows a comparison of the IR spectra for
diastereomers DiBr-I, DiBr-II, DiBr-III and DiBr-IV in KBr,
which are summarized below in Table 3.
TABLE 3
Band, cm'' Fmnc~tinnal Groins
3500, 1105, 1070 tert. and sec. OH
3420, 1670, 1580 -CONH-
3110, 3060, 1605 monosubs. aromatic
1505, 770, 710 rings
2915, 2870 -CH,-; -CH2-; -CH- in
2960
, aliphatic or cylic
1465,1370
comps.
3020, 1670, 1310 double bonds
980
730, 1270 aromatic esters
1715, 1240 ~c=o
1730, 1180 acetates
855 oxetane rings
FIG. 5 is an EI mass spectrum of diastereomer DiBr-
I which is the same fragmentation pattern for diasteromers
DiBr-II; DiBr-III and DiBr-IV, and is summarized below.
~i
CA 02210924 2002-09-06
_ 28 _
FIG. 5 EI-MS; (M]'=992 !m/z; the main fragments)
568 (T] '; 550 [T-H~OI '; 508 [T-AcOH] ';
490 [T-AcOH-HZO]'; 448 (T-2AcOH]';
or [T-BzOH]'; 390 (S-0-HZO]';
386 [T-AcOH-BzOH]'; 348 (S-0-CO-HCHO]';
326 [T-BzOH-2 AcOH]'; 308 [T-326-HZO]';
284 [327-Ac]'; 264 [832-T]'; or
(424 - 2HBr]'; 246 [264-HZO]';
218 [264-HCOOH]'; 188, 167 [S-CSHa0NBr2]';
148 [167-Hz0]'; 122 [BzOH]'; 105 [8z]';
91 [C.,H,] '; 83 [C,H,C~O] '; 77 [C6H5]
'; 57, 55 .
(T = taxane ring in the compound;
S = acid (side) chain in the compound.)
FIG. 6 is a FAB+ mass spectrum of diastereomer
DiBr-I which is the same fragmentation pattern for
diasteromers DiBr-II; DiBr-III and Di.Br-IV, and is summarized
below.
FIG. 6 FAB' - MS: (positive ion mode)tm/z)
1030 (M + K] '; 1014 [M + Na] '; 992 [M + H] '
(See Elem. Anal.); 974 [M-HZO]'; 932 [M-
AcOH] ' ;
914 [M-AcOH-Hz0] ' ; 912 [M-HBr] ' ; 870
[M-BzOH]'; 854 [870-Hz0-2H]; 832 [M-2HHr]';
705 (M-243- Ac]'; 569 [T]'; 551 [T-Hz0] ;
509 [T-AcOH] '; 491 [T-AcOH-Hz0]'; 448 [T-
BzOH]';
429; 424 [SH2] +; 413; 405 [S- HZO]'; 391
[S-0-Hz0] '; 387 [T-AcOH-B20H] '; 376 ; 347
(S-0-CO-HCHO]'; 338:327 [387-T-AcOH]';
315; 284(327-Ac]',279; 264[832-T]' or
(424-2HBr]'; 246 (264-HZO]'; 231; 218 [264-
HCOOH] ';
188; 167 [S-CSHBONBrz]'; 149 [167-H20]';
133; 122 [BzOH]'; 113:105 [Bz]'; 91 (C,H,]';
83; 77 (C6H5]'; 76; 57; 55;
(T=taxane ring in the compound; S-acid
(side) chain in the compound.)
i i i ~ i
CA 02210924 2002-09-06
FIG. 7 is the 'H NMR spectrum for diastereomer DiBr-I.
FIG. 8 is the 1H NMR spectrum for diastereomer DiBr-II.
FIG. 9 is the 1H NMR spectrum for diastereomer DiBr-III.
FIG. 10 is the 1H NMR spectrum for diastereomer DiBr-IV.
FIG. 11 shows the 1'C-NMR spectrum for diastereomers
DiBr-I, II, III and IV. The 1H NMR and 1jC-NMR spectra for each
diastereomer is summarized below:
lii DIBr-I 'H-I~t in CDCL,
(300 hgizin DDm: side chain and s ig~ortanr r..r,r.,ws
only)
Chem icalShift om) Ass~g~menre
(n
3.36 (d,1H)- (~Q - C - C= O)(HO - 2'a)
r
15 i
4 . (d,1H) - (#j - C - C = O) (HO - Zp' )
74 r
r i
. (d,1H) - (N - ~ - ) (H - 3' )
68 i
4 .62 (qt, 1H) (>~ - Br) (H -3")
-
20
1
1.81 (s,3H)- (Br - C -
i ~3) (3H-4")
r
1 . (d,3H) - (Br - C - Q~~) (3H-5" )
78 r
r
25 - C = O
2.35 (s,3H) - (- 0 - C - ~~) (3H-4)
0
2.68 (m,1H) (-
S~ -) (H - 6a)
1 .98 (m,1H) - (- ~ -) (H - 6a)
30
4.41 (m,1H)- (- ~ -) (H - 7a)
OH
2.46 (d,1H) - I- C - ) (H - 7b)
...
35 6 .28 (s,1H) - (- g~ - O - OCCH~) (H - 10)
r
2.22 (m,2H)- (- ~ - )(2H - 14, a, b)
2 . (s,3H) - (- ~) (3H - C - H)
O1
4.22 (qt,2H) (- g~ - )(2H - 20a, b)
CA 02210924 1997-07-21
- 30 -
DIBr-I 1'C-NMR
300 Ngiz in ppm; side chain
and some important carbons only
Chemical Shift (ppm) Assicrnments
5 170.3 - (C - 1'; C = O)
73.0 - (C - 2')
54.6 - (C -3')
172.4 - (C - 1"; C = O)
70.1 - (C - 2")
10 55.4 - (C - 3")
22.7 - (C - 4")
27.6 - (C - 5")
203.5 - (C - 9; C = O)
CA 02210924 1997-07-21
- 31 -
DIBr-II 1H-Nl~t in CDCL3
(300 l~iz in ppm: side chain
and some important protons only
Chemical Shift (ppm) Assictnments
i i
3 (d,1H) - (HO - C - C = O) (HO - 2'
. a)
42 i
i
4.74 (d,1H) - (- HC - C - O) (H - 2b)
i i
i i
i i
5.68 (d,1H) - (- N - CH) (H-3' )
i
i
4.62 (qt, 1H) - (>CH - Br)(H- 3")
i
i
5 .81 s, H) - (Br - C - CH3) (3H- 4")
i
i
1. (d,3H) - (Br - C - CH3) (3H- 5" )
78 i
i
- C = O
2.35 (s,3H) - (O - II - CH3) (3H- 4)
O
2.68 (m,1H) - (-CHZ -) (H - 6a)
1.98 (m , 1H) - (- CHZ -) (H - 6a)
i
4.41 (m,1H) - (- CH) (H - 7a)
i
i
OH
2.48 (m,1H) - ( C - ) (H - 7b)
i
OH
6.28 (s,1H) - (- CH - O - OCCH3 (H- 10)
i
i
1
2.22 (m 2 H) - ( - CHz -) (2H - 14a, b)
,
2.01 . 3H) - (- CH3 -) (3H - C -18)
(s ,
4.22 (qt2H) - (- CH2 - ) (2H - 20a,b)
,
CA 02210924 1997-07-21
- 32 -
DIBr-II 1'C-NMR
j300 N~iz in ppm; side chain
and some important carbons only
Chemical Shift (ppm) Assicrnments
170.3 - (C - C = O)
1';
72.9 - (C -
2')
54.6 - (C -
3')
172 . 4 ~ - (C - C = O)
1"
)
70.1 - (C -
2")
55.2 - (C -
3")
22.7 - (C -
4~~).
27.9 - (C -
5")
203.5 - (C - C = O)
9;
CA 02210924 1997-07-21
- 33 -
DIBr-III 1H-NMR in CDCL3
(300 N~iz in npm; side chain
and some important protons onlv
Chemical Shift (pt~m) Assignments
i i
3.23 (d, 1H) - (HO - C - C = O)(HO 2'a)
4.76 (d, 1H) - (- HC - C - 0) (H - 2b)
i i
i i
i i
5.65 (d, 1H) - (- N - CH) (H-3' )
i ii
i t
4.62 (qt, 1H) - (>CH - Br) (H- 3")
i
i
15 1.98 (s, 3H) - (Br - C - CH3) (3H- 4")
i
i
1.28 (s, 3H) - (Br - C - CH3) (3H- 5")
i
i
- C = O
2.45 (s, 3H) (0 ~~ CH3) (3H- 4)
O
1.72 (t, 2H) - (- CHz-) (H - 6a,b)
3.72 (m, 1H) - ( - CH -) (H - 7a)
25 i
OH
4.62 (s,1H) - (- C -) (H - 7b)
t
i
OH
6.79(s,1H) - (- CH-) - OCCH3)(H-
i 10)
i
2.42 (m,1H) - (- CHZ -) (2H - 14a,
b)
2.05 (m,1H) - (- CH2 -) (2H - 14a,
b)
2.18 (s,3H) - (-CH, - ) (3H - C -
18)
4.38(s,2H) - (- CHZ - ) (2H - 20a,
b)
CA 02210924 1997-07-21
- 34 -
DIBr- I I I 1'C-NMR
(300 NIFia in perm: side chain
some important carbons only)
Chemical Shift (ppm) Assignments
169.3 - (C - 1' ; C = O)
72.9 - (C - 2')
54.0 - (C - 3')
172 . 5 - (C - 1" ) C = O)
57.7 - (C - 2")
54.5 - (C - 3")
22.6 - (C - 4")
29.4 - (C - 5")
207.1 - (C - 9; C = O)
CA 02210924 1997-07-21
- 35 -
DIBr- IV 1H-NMR in CDCL3
(300 I~iz in ppm; side chain
and some important protons only)
Chemical Shift (ppm) Assignments
S i i
3 . 23 (d, 1H) - (HO - C - C = O) (HO - 2' a)
4.76 (d, 1H) - (-HC - C = O)(H - 2b)
10 i
5.65 (d, 1H) - (-N - CH) (H-3' )
i ii
i i
4.62 (qt, 1H) - (>CH - Br)(H- 3")
i
i
15 1.98 (s, 3H) - (Br - C - CH3) (3H-4")
i
i
1.28 (s, 3H) - (Br - C - CH3) (3H- 5" )
i
i
- C = 0
20 2.45 (s, 3H) (0 ~~ CH3) (3H- 4)
O
1.72 (t, 2H) - (- CHz -) (H - 6a, b)
3.72 (m, 1H) - (- CH -) (H - 7a)
25 i
OH
4.62 (s,1H) - (- C -) (H - 7b)
30 OH
6.79 (s,1H) - (- CH-O - OCCH3)(H-
i 10)
i
2.42 (m,1H) - (- CHZ - ) (2H - 14a,
b)
2.05 (m,1H) - (- CHZ - ) (2H - 14a,
b)
35 2.18(s,3H) - (-CH3 - ) (3H - C -
18)
4.38 (s,2H) - (- CHz - ) (2H - 20a,
b)
CA 02210924 1997-07-21
- 36 -
DIBr-IV 13C-NMR
(300 Ngiz in ppm; side chain
and some important carbons only)
Chemical Shift (ppm) Assicrnments
5 169.2 -( C-1'; C=O )
72.1 -( C-2' )
54.1 -( C-3')
172.5 -( C-1" ; C=O )
57.8 -( C-2" )
54.3 -( C-3" )
22.6 -( C-4" )
29.4 -( C-5" )
207.1 -( C-9 ; C=O )
Physico-chemical properties of dibromocephalo-
15 mannine/7-epi-cephalomannine diastereomers of this invention
are summarized below in Table 4:
CA 02210924 1997-07-21
- 37 -
TABLE 4
Physico-Chemical Properties
of Bromo-Analogues of Paclitaxel
Property DiBr-I Di-Br-II DiBr-III DiBr-IV
Appearance Off-white Off-white Off-white Off-white
to to to to
slightly slightly slightly slightly
yellowish yellowish yellowish yellowish
crystals crystals crystals crystals
Melting 185-187C 171-173'C 166-168C 163-165C
point
Molecular Cq5H53014NBr2Cq5H53~1qNBr2Cq5H53~1qNBrzCq5H53~1q~r2
formula
Molecular 991.7 991.7 991.7 991.7
weight
[]D -41.3 -44.4
IR*(cm-1) 3500, 1105,
1070; 3420,
1670, 1580;
3110, 3060,
1605, 1505,
770, 710;
2960, 2915,
2870, 1465,
1370; 3020,
1670, 1310,
980; 1730,
1270; 1715,
1240; 1730,
1180; 855;
W ~,~X;(E) 226.0 nm; 226.Onm; 219.4 nm; 218.4 nm;
14732 12415 37900 20013
TLC** (Rf)
solvent
systems: 0.34 0.37 0.63 0.65
A
. B
0.28 0.30 0.54 0.57
HPLC***
(RT)
condition
1: 43.81 min. 45.01 min. 69.68 min. 71.92 min.
condition
2: 46.65 min. 48.39 min. 69.66 min. 72.60 min.
* The IR spectra of DiBr-I-IV are superimposable.
I I ~I I
CA 02210924 2002-09-06
- 38 -
** Solvent System A: Methanol-1,2,-Dichloroethane-
either (1:9) or (1:10).
Solvent System B: Hexane-Chloroform-Ethyl Acetate-
Methanol-(2:6:1.5:0.5)
*** Condition 1: Column: ES'~ Industries FSP
(Pentafluorophenyl) 4.6 mm ID x 250 mm, 5 um particle size,
60A pore size; mobile.phase - water - acetonitrile - methanol
- (41:39:20); flow rate 0.50 ml/min; separation mode -
isocratic; detector - Waters 990T"photodiode Array Detector;
elution monitored at 227 nm; injection volume - 20 u1.
Condition 2: Column: Phenomenex 4.6 mm ID x 250 mm, 5 um ,
particle size, 80A pore size; mobile ghase - water -
acetonitrile - methanol - (45:40:15); flow rate - 0.50 ml/min;
separation mode - isocratic; detector - Waters 490
programmable multiwavelength detector, elution monitored at
227 nm; injection volume - 80 ~1 total mixture.
EXAMPLE 5
In Vitro and In Vivo Studies Showing
Antitumor Efficacty of A Mixture of Dibromo
Cephalomannine/Dibromo-7-epi-
Cephalomannine Diastereomers Which
Correlate to Known Paclitaxel Antitumor Efficacy
As is known, paclitaxel and its derivative Taxotere'
(Rhone-Poulenc Rhor) exhibit highly desirable antitumor
efficacy against a number of tumors. These antineoplastic
drugs act in a unique manner by preventing depolymerization of
tubulin forming microtubules of the mitotic spindle which is
essential for cell division, and thus cause cell division to
cease along with tumor cell proliferation. The mechanism of
action of paclitaxel, its pharmacology, etc. is described, for
example, in Rowinsky et a1. Taxol: A Novel Investigational
Antimicrotuble Agent, J. Natl. Cancer Inst., 82:1247 (1990).
In accoradance with this invention, a mixture of
novel dibromocephalomannine/dibromo-7-epi-cephalommanine
diastereomers has been found to exhibit strong paclitaxel-like
antitumor efficacy in vitro and in vivo.
i i i ~ i
CA 02210924 2002-09-06
- 39 -
5.1 Ia Vitro Studies (NCI)
The following in vitro studies were conducted by the
National Cancer Institute's Developmental Therapeutics Program,
which demonstrate strong antitumor efficacy of the inventive
dibromocephalomannine diastereomers which efficacy correlates
closely to that of paclitaxel.
The Developmental Therapeutics Program provides as a
service to the public an in vitro anticancer drug discovery
screen using a panel of sixty different human tumor cell lines
over which candidate drugs are tested at defined ranges of
concentrations. See Boyd et al., Drug Development Research
34:91-109 (1995). As discussed in Boyd et al., the screen is
designed and operated in such a manner that both relative and
absolute sensitivities of each of the cell lines comprising the
screen are reproducible to the degree that a characteristic
profile ("fingerprint") of a respective cell lines' response to a
drug candidate can be generated. Recent studies of the in vivo
counterpart of the NCI in vitro screen have indicated the in
vitro screen to be an effective selector of compounds with in
vivo anticancer efficacy. See Greyer et al., Proc. Am. Assoc.
Cancer Res. 35:369 (1994). Operation and interpretation of the
screen are discussed in detail in Boyd et al., as well as in
several other articles cited therein and thus need not be
repeated here, except comparative results obtained from the
screen between the novel 2"3"- dibromocephalomannine/dibromo-7-
epi-cephalomannine diastereomic mixture represented as compound
"XCLY-401759 analog" and that of the known antitumor compound,
paclitaxel. Table 5A-5F shows the data sheets of in vitro testing
results of a mixture of dibromocephalomannine diastereomers-I and
-II in a screen of sixty human tumor cell lines. Figures 12A-12F
are mean graphs of dose response of a mixture of
dibromocephalomannine diastereomers-I and -II in a screen of
sixty human tumor cell lines. Figures 13A-13F are means graphs
of dose response of paclitaxel in a screen of sixty human tumor
cell lines.
CA 02210924 2002-09-06
- 39a -
U7MM~t00 Q~N Lf7~-~O~MvD~ I~~-~M.-..--.(~
O r~ 00 00 ~D V Q~ U7 cLl l~ o pp .-~ ~ o .~ o o U7 0
M (U (L1 f~ M ~~ CLJ O O O O O O O .-. O O O O
I O O O O O O O O O O O O O O O O O O O
C I
O
._ ~ ~ I~ ap O dwD vD 0~ f~ ~-~ 0~ I~ .~ Ilk M tf7 CU W D
111 o OW D ~" O~ I~ tI7 O O .-. U7 pp .-, O Q~ (L1 00 M v0 ~ ('~
N (U ~ I~ (U I~. 1~ I~. (7W D ~ 00 (L1 --' .-~ ~ M o M ~t
-+-' ~ I 00000 000000000 000000
._
N
a I~ M ..., .~ 0 00 ~ M U7 t~. 00 vD ~ tf7 I~ f~ (U ~ o U7
C ~ O d- M o 00 .~ M t\ (U (U M 00 .-~ .-~ t17 0~ t\ 00 II7 .-~ ~r
U f~ ~ MMIf»M f~f~I~W DMO~(U~ o~?'tUOM~
I 00000 oo0ooooo0 000000
_ d
U Lf7 ~t ~ .~ U7 0~ f~ ll~ (U U7 ~t vD I~ 00 ~ O U7 vD t~ In
O +~ o ~ ~ CO --~ (Ll ~ QWD ~ 00 I~ (U N M o U7 o O~ .-. Iw
J a - MMtf~ -M I'~OpI~W pMQO(LN --~tl~MOM~
_ ~
I 00000 000000000 000000
C
d _
L!'7 vD ~ (U U7 lf7 I~ (U tf7 ~ WD 00 0~ ~ Q~ M o t\ U7
MMvDO~M 00~(LI~(WJ7oMM .-.I~~-.~M~t
. . . . _ . . . . . . . . . . _ . . . .
I O O O O O ...-. O .~ O O O .-~ O O O O O O O O
(LI.-~I~00vD ~M00~lf7vDMv0OW DCUovD00Q~
E~-i -~ tn I~ (U d- U7 00 M (U ~ U7 ll~ 00 ~' M I~ o M CU 0~ In
L 0~ ~- M n o p~ .-, ~ .~ .... ~- tf7 v0 O~ (U vD (~ 0~ CLI (U
. . . . . . . . . . . . . . . . . . . .
O .--~ .-r .~ .--~ L .~ .--~ .-r .~ .-. ....v .-~ .~ O .--~ .-~ .--~ O ..-~ .~
DJ
U
O M M ~D .-~ C M DO .-~ f~ M ~ (U Q~ N (L1 CU ~ o o ~
O vDM~tO~M d~0~~-~~-~J 000~t1W 0 (Ut\~tO~MM
L (U~tMt~.MUMMvDf~lf~Mtl~.-~~ (UM.-~o(U.-.
._ <y . . . . . . . . . . . . . . _ . . . . .
L..- N 00000 ~o00ooo0o0 000000
C
J
C
J
N L
-' ~ ~D U U
-- ~ t~C7 CLJ U vD CU o CU U IW D
QW.~ H CU -~ ~ CtJ M CU ~D CU C O W D
U U ~ ~- M J Q CLJ CU CU CU M d- U7 d CU OW O o
\ d I o I I d \ ~D ~ I 2 I Z I U CU --~ (1l
~ l.i 'D I- ~ E Q~ X I I I I I I I O I I ~ CU 'D
N ~ I J ~ c~ V~ > ~ ~ ~ ~--~ ~~ ~ ~-.~ C J U I- CU ~ I
~ ~ U J D ~ Q' I lt7 ~C O D U U U U U O O U U I- ~ 3
w C 5 U Z ~ ~ v7 C Q !J I I Z Z Z Z Z J U I Z 2 ~C t/~
d ~ O O
~J Z U
I ~ i
CA 02210924 2002-09-06
- 3 9b -
U7 In I!~
U7 U7
~ ~i- c- v ~t ~ ~Y l!7 lf") U7 - 0 0 0 0
LI7 lf7 Lf7 I~ 0
O O O O O O O O O O O O O I I I I I
O
1 I I I I I I I I I I I I ~L,J W W
I W w
LvJ l~J LJ L~J L~J LJ L~J L~J L~J lf7 I~ O
L~J L~.! L~J LJ L~J (Y7 ('~
O O O O O O O O Q1 ~ [w (W (~
.~ .-r
[j~OOOOO OO~~~[,(~~p~(u
U . . . . . . . . . . . . . ~ M ,....,
.
J ~ ~ ~ ~ ~ ~ ~ t~ M ~ M ~ M
n n n n n n n
~t 00 Lf7 lf7 U7 Lf~ U7 U7 U7 00 U7 U7
lf7 tf~ 00 t\ U7 U7
O O O O - O O O O O . O O O O O O O
I I I I O O
I I I I
I I I I I I 1 I I
LJ L~J LJ L~J LaJ LJ LJ LJ L~J I
W L~J L~J L~J lsJ L~J
L~J LJ L~J
ooo~ ~.-.Nppo (Uppo oorT~(~7~
.-.ooo~ M~N~~ ~poo o(~(vo~~p
nv v
00 00 00 Cb QO W D 00 00 00 00 C~ 00 00
00 00 CO 00 CO 00
00000 000000000 000000
I 1 I I I
1 I I I I I I I I I I I I
p4 W W w L~J LJ I I
W W W W LJ L~J L~J W W W W VJ
VJ LJ W
000000 opp~oooo00 000000
~ooooo o..~~p'pooooo 000000
p4 ~vvv~ v ~vvvv ~~~v~v
00~11~1~(''7CU(Ul~ol~f~00~DoU7 oo00QW D~
-~-('~--~ ~('~00~000~00Q~~ of~O~Q~I~Q~
I
I I I I -~ I I I I I --~ I I I
I I I I
I I
ovDvD00~00 Il~(U~oO~Q~('~MO~ ~-~CU--00(~
S - C'7 ~ CU ~t (U --, (1J ~' ~--~ ('~
lf7 (L1
C .f.~Lf7 I I I I I
O 3 I
-- O
~
oLf7MvDovD ~f'.-..~
d t~ ~ l!~ o ('7 WD ~D CO 0~ o
I~ I~
L . ..~ CU (U U7 .~ ~ ~ CU tl~ ~ CU
.~ .~ I vD
.E.WD I I I I
C C I
Q~
C ~ o c- 00 ~ 00 00 lI7 U7 c''7 ('~ d- o .~
(''7 CU ~ ~t tf7 00 00 0
O ~ - ~' ~ N (L1 vD - ~ CU I ll7 -~ .-~
.~ I N ~- (''7
U ~ 1~ I I I
I
o
o ~"~ ~ c- ~ ~ o vD ~ c~ f~ c''7 U7 (r7
~r C'7 Lf7 ~r cL! c~ ~
_ _. O - ~ ~~ C'~ ~ IW D lf7 ~ ---' M C''7 .-.
.~ V' ~ -~ .-. CLI
J ~ I I I
I
CA 02210924 2002-09-06
- 39c -
U7N~~rNo vwl~o~~00-- wDNO~~~~
I~Mo0~0~~ ooNMd-.-..-,.-. ~o.....,NN~
oo.-,NNo 00000000 0000.-~.-.
000000 00000000 000000
I
MCOOVOIn~ M~MMf~00o~ U7l~NvD~tln
MOMNIf~M vDIW DoI'~N~M OO~OOI~MN
U7 lf7 I!7 00 o tf7 N vD 0~ I!7 N !I7
U7 M M 00 v0 M M U7
000000 00000000 000000
oQ~.-~.~~o I~MU7--~Q00~.-~v M.--~W DvDo
NOOOIW D~ N~MNOLf~U7U7 O~wDU7NN
vDtf~Mt~MM ~~--~~ONOOII7 lf~NvDMMtf~
O O O O O O O O O O .-~ O O O O O O
O O O
I~OOI~f~M.-. .-.vD.-.ooM~o ~7-I~O~~~rO~
OONN~n00 1I700~~tU7ll~MvO M~OOOvD~
vDMIn~~M ~tlf~M~0~N00v0 vDNvO~MLf7
000000 00000000 000000
U
I~ .-~ I~ 0~ 0~ f~ 00 N -~ M ~ 00 --~
00 M U7 U'7 I~ .-~ N
CO M vD f~ ~f 0~ M U7 --~ 0~ ~ 0~ O lf'7
vD ~ O~ ~ 0~ -
W I~ M I~ .- I~ U7 vD (W O Q~ f~ N I~ ~
~ M 00 ~ ~ I~
. _ . . . . . . . . . . . . . . . .
. .
f~ or..o.-,o0 00000000 000000
OO~OONNN OOIf--~OOo~N ~tnMvDNM
-Nt~tf~O~Lf~ NO~OOO~vovDo tf7M0~~t~M
NOOOW DON MQ~N~Lf7NMll~ NOOOOo~~
.-, O .~ ..-. .-. .-. .-~ ..~ .~ O O .-, ....» ~,
vDNl~~f~tf7 ~MtI7NW DI~O MN~M.-~~
MvDO~NLf~Q~ I~.MU7~~-~OOII70~ i~l~--~O~M00
~Mlf7~ll7.-~ OMN~tU7OU7M MNvNN~t
000000 00000000 000000
U
00 c
L ~--'~ NNLf~n d
Q> > M t I I III N U ~ M ~ U7 CO M
U CO U7 01 Q~ fI~ d ~ I J J J N vD > I I I I I
c vD ~ M .~ t~ ~ ~-~ IsJ LsJ i.~.l 1,,~ 1 I C p ~ ~ Q! ~ >
d N N Ll~ I I .~ O ~ ~ ~ ~ U U d I Q Q Q Q p
U I I I C~ Oa U7 C X J ~ I I I U U -- ~ U U U U I
L~ l.L L~ Z Z CU d p Q ~ Y Y Y Q Q L l~ > > > > Y
__. ~~~~~~~J J~~~N~~~ d~ppppc~
Z v >
U ~ p
~.~, Ii ~ ~I
CA 02210924 2002-09-06
- 39d -
U7 lf7 lf~ v U7 U7 U7 U7 U7 U7 U7 U7
~t I!~ Ln U7 U7 U7 ~ U7
000000 00000000 000000
I I I I I I I I I I I I I I I I I I
w w W LJ L~J I I
w W W W W w W VJ W W W W W
W W
NNvDoovD c'~o~aOvDtf70DW Dt~00vOoM
tl~ O~ Q' o 0 U7 ('7 ~D Q~ 0~ -~ OD ~ 00
00 .~ U7 0 O .-.
~('~(''7--,~M CU4'('~C'~~t-U7~td-U7N~('~-vD
a
U7 l(') 00 U7 ~D In U7 if ) U7 00 U7
0p U7 U7 U7 U7 U7 U7
O O O O O O O O - O O O O O O O O O
O
I I I t I I I I I I I I I I I I I
WWWWWW WW WWWW I
I~wWWWW
U7 U7 ~ OD ~ wD U7 ('~ ~t Q~ o OWO 00
d N ~ o
c''7 N M .- o 00 00 -w0 0~ l!~ I~ ~ 00 N
M U7 (U
.-. .-.. ('~ C7 .~ N .~ .~ r.. .~ .-.
M U7 ~--~ ...a .-, .-~
.~
QO CO ~ 00 00 00 00 00 QO 00 00 00 vD
CO CO 00 OD 00 00 00
o O O o O O o O O o o O o O o o O O
o o
I I I I I I I I l W I I I I I I
wwwwww wwwwwwww I
wwwwww
od-o~oo 00000000 oo~pooo
o~o~o0 00000000 00000
.~ N .~ M .-. .-. ..~ .~ .-.. .~ ~, ~ .-...
...., .-. .-a ..-~ .-. .-.
.--~
~ vv vvvv~vvv vv v~v
H
('7.-(~MQ~U7 In00~'(U~I~I~ OOW D('~~In
0~ ~ 00 (''7 0~ 0 0~ ~ 0~ t\ 00 0~ 0~
~f 0~ 0~ ~ 0~ ~t vD
I I I I I I I --~ I I I I I I I I I
I I I
I
No~f~o~ o~.~~U7N--~N ~~U7--r-wD
N (Y7 .- V' N N I M --~
I ~ N .-.
I
~tovD~vW Dhf~~f00U7vDlf7 U7f'~NODoI>7
c1! --~ M CLJ CU .~ ~t .-. ~- N cU It7
c'~7 -- ,~ ('~ .-. I
I I I I
cUNNoU7C0 000--Nlf7lf7~ o~r~tnU7I~
(~ C'~ ~ ~ .- M C'~ - -. ~1' M I In -~
- -~ C''7 N .-~ .~
I a I
Lf7 vD M CU vD CU vD Q~ fW D 00 ~1- -~
U7 (''~ N ~ -r 00 l!'7
_ ~- ~ .mD C~ CU ~ c ~ ~ ~r N ~ C'WD N cLl
_ c~7 (r7
.
i~., ,, ~'i
CA 02210924 2002-09-06
- 39e -
0~ M o t'~ 00 ~D ~D M f~ I~ 0 ~D
~D (U ~ (L1 ~ I~
.-. p~ .~ O (~ ~ ~ O o o <n (U O
o 'p o .-. ~
o O O O (~ o O O O .-. O ~ .-.
O O O
O O O O O O O O O O O O O O O O O
O
I
N I~ Lf7 In CO D 0 CO M ~' N M QO
N n .w ~i' M
~t ~ 0 00 (1J ~ o Lf7 U7 ~ ~ ~ d- 00
M o vD 0
(L! -~ Q~ ('~ dwD v0 wD (U U~ ~- o
00 vD C'7 Lf7 vD
o.-000000 00 00o0oo.-,O
(L1 (L1 ~--~ Lf7 ~ U7 ~ I~ U7 0 0~
0~ Il~ (L1 ~ Ll7 vD M
~00(LJOU'7d-OO ~0~ W DII~OI~MOIn
NOW DCW DOT lf~(1J~o(UU7OOU7vD
00000000 00 O.--~oooooo
O~Il~OMOW D~C- ~~ II~~fUO~('~t'~00~
U7 o r\ N 0~ r' ~ N U7 r~ Q~ U7 tf7
~ .- U7 0 r~
W (''7 .~ .-. pp ~D --~ .-. (U tf7
~t vD O O ~ O O IW D
~
O .-. .-. o o o O O O .-. O O O O
.~ r. O o
a
M
0~ ~ ~iwD tI7 00 dl ("~ n N I~ t~
~t o o U7 00 ('~
~cU~OWDI~(U~ COvD ~NW Dool~l~
o.-..-~ooo.-..-. 00 0.000000
00(''7~MMvD00(LI IW D ~t.--~W DU7vD('~0
vDNCllo(lJvDl~o ('~~t ~O.-~(~OOvDll70
~DUW OoOvDCU~ fwD lI7(UvO~--~~'.--~c~7
O .-. .-. .-. .-. .-. .-. ..~ ~ .~ O ,-. O .~ .-.. .-... .-. .-.
--r U~ Wit' ~ (1J ~t I~ ~' o (U vD U'7 U7 vD I!~ 0~ CU 0~
nn~IW DIW D--~ ~'--~ 00U7~0('W D(LI~D('"7
n ~f (W T (~'7 ~D ~ ~' M --~ ~ CU 1D ~ CU ~ ~
O O O O O O O O O O O O O O O O O O
U
U
i t
N Q
U i LJ \
L C ~ ~ --~ U7
N d U I C''7 M
U U C ~ (L1 V'
C C''7 d f~ I H- I
d ~0~ N U7U QG'~00~7
U 0 I ('7 U o --~ +' ~' \ f I~ ~ Z C' f~
I ~ Z ~ I (U ~~ ('~ d ('7 ~-~ +' I~ I'~ I Lf7 I I U7 I~
-W D Q~ _ ~L L~ ~~ 1 -1-' I I 111 li L.L a Q Q I ~
d 00 ~ U Q X Z Y O U1 U Z d U U ~1 c~ La G~ f- I
c I~ Q Q U ~ (n F- Z O Q. ~l ~ ~ ~ ~ I ~ ~ a7 H
-Oi i i
Q' ~ fuel
I i I~ I
CA 02210924 2002-09-06
- 39f -
U7 Lf7 Il~ tf7 U7 U7 U7 tI7 II7 ~
U7 ~ U7 II7 CO lf~ Lf7
00000000 00 0000-000
I I I I I I
1 I
I I I I I I I I I
~J W W W W ~J W W w ~J L~J W W
W W W W
O ~ ~ ~ O ~ O ~ ~ (U p~ ~ O O ~
Q1 ~Q
CU ~ ~i' (U (U ~ (U D V' ~ O O ~
O O IW c)-
W D ~' M M M -' ~ (LJ Lf~ (L1 ~~
~ ~ ~ M V
a ~ a
U7 U7 Il~ U~ I~ U7 00 U7 (~ 00 Op
In lf7 LI7 0p Lf7 U7
O O O O O O O O O O O O O O O O
I I - O
I I
I
I I I I 1 I I I I
I W 1
I LJ W W VJ W W
~J W W l.~.l W LEI VJ W
LEI VJ
~OMM(U~~f'o~ ~ Ot\OOU7o0[f~.-...~
M d~ 0~ O vD ~t M O f~ I~ V O O
VW D M U7
v vv
~ 00 n I~ 00 00 00 N vD 00 00 00
t' I~ 00 00 00 00
00000000 00 00000000
I I I I I I I I I
I I 1 I I I I I
LJ W LJ W W W W W 1
VJ LEI w W W W W W W
w
MOOM~oor7pp o0 o~pooooo0
U7pp(,f~ooo~ppp o0 o~pOOOOOo
W M U7 M ~ --r --~ ,.~ .~ M ..-. .-.
M vD .-. ..~ .~ .,...,
.-,
v v ~ v ~ ~ v
H
QWOM00(U~If7o ntl~ 00(~I'~~tovDn
MI~O~~O~tT~t'o OOt~ ~t~0~(U(Uo~~
I I I I I I I --~ I I I I I I ~ I
I I I
I
~o~.~I~00001~ MM lf~U-7vD~MNIf~O~
In M I .--~ N (U .--~ --~ (U I .~ .--wD
.-~ .~
I I I I
~t 00 o vD CU --~ --wD ~ (U ~t .-~ (LJ
00 M U7 vD lf7
CU ~Y M (1J CLl -, (Ll 0~ I (L1
M ~J' I lf7 00 (L1
I I I I I
00 ~t N --~ I~ M vD tW 0 00 (U vD
vD I'~. 00 fW 0 I~
M Vw0 f~ (U vD 00 ~ --~ 0~ -wD I~
M (U
I I I I
U-7oU711~11~--~~o oN ppol.f~00MV~I~U'7
vDt~00Q~MM~o MN N~~ U7n~'M
I --~ I I
II I
CA 02210924 2002-09-06
- 40 -
5.1.1 Discussion of Results (NCI)
In the NCI in vitro anticancer drug screen the
effect of an antitumor candidate, i.e. XCLY-401759 of the
present invention, on a cell line, percentage growth (PG), and
calculated response parameters are discussed in detail in Boyd
et al., Data display and analysis strategies for the NCI -
disease -oriented in vitro antitumor drug Screen, Cytotoxic
Anticancer Drugs: Models and Concepts for Drug Discovery and
Development, Kluwer Academic Publishers, Amsterdam, pp. 11-34
l0 (1992), and Monks et al. Feasibility of a high-flux anticancer
drug screen utilizing a diverse panel of human tumor cell
Lines in culture, J. Natl. Cancer Inst. 83:?57-766 (1991),
In general, in the screening data report, Tables 5A-5F, and
mean graphs, FIGS. 12A-12F and 13A-13F, "GISO" represents the
50~ growth inhibition factor, "TGI" represents a total growth
inhibition, or cytostatic level of effect, and "LCso"
represents a lethal concentration, or net cell killing or
cytotoxicity parameter. Values accompanied by a "<" signify
that the dosage level or real value is a value that is
something less than the lowest tested concentration, and
values accompanied by a ">" sign indicate that the effective
dosage or real value is a level greater than the highest
tested concentration.
The mean graphs are obtained from Glso, TG.I and LCSo
concentrations obtained for compounds tested against each cell
line in the NCI in vitro screen. A detailed discussion of
mean graph construction is provided in Boyd et al. (1995). In
interpreting the mean graphs, in general a bar projecting to
the right represents sensitivity of a particular cell line to
an anticancer candidate in excess of the average sensitivity
of all tested cell lines, while bars extending to the left
represent cell lines which are less sensitive on average to
the anticancer candidate. The bar scales are logarithmic,
such that a bar which extends, for example, 2 or 3 units to
the right of the vertical reference line in, say a GIso mean
graph, indicates that the anticancer candidate achieved a
response parameter for a particular cell line at a
i ~ I
CA 02210924 2002-09-06
41 -
concentration one-hundredth to one-thousandth of the mean
concentration required over all cell lines, thereby indicating
that the particular tumor cell line is unusually sensitive to
the tested candidate.
Turning now to FIGS. 12A-AF, XCLY-401759 shows a
relatively high magnitude of effect in TGI, for example, on
Leukemia cell line HL-60(TB); Non-Small Cell Lung Cancer line
NCI-H522; Colon Cancer cell lines COLD 205 and HT 29; CNS
Cancer cell lines SF-539 and SNB-75; Ovarian Cancer Cell line
OVCAR-3; Renal Cancer cell line RXF-393; and Breast Cancer
cell lines MCF7, MDA-MB-231/ATCC, HS 578T, MDA-MB-435 and MDA-
N.
In comparison with FIGS. 13A-13F, anaylsis of paclitaxel,
XCLY-401759 demonstrates an unusually high magnitude of
response such as that of paclitaxel to Non-Small Cell Lung
Cancer cell line NCI-H522 (<-8 v. <-10 for XCLY-401759 and
paclitaxel, respectively). Compare also the respectively high
magnitude of response of both XCLY-401759 and paclitaxel on
Colon Cancer Cell line COLO 205 (<-8 v. -7.97); on CNS cancer
cell line SNB-75 (-7.30 v. -9.18), and, for example, on Breast
Cancer Cell line HS 5787 (-7.61 v. -9.91).
The high magnitude of effect of XCLY-401759 on many
cell lines is perhaps more pronounced in GIso in which XCLY-
401759 demonstrates a high response level in many of the same
cell lines as does paclitaxel, such as, for example, with
various tested colon cancer cell lines, melanoma cell lines,
ovarian cancer cell lines, and renal cancer cell lines, and
thus falls within the footprint of paclitaxel-like antitumor
activity thereby reproducibly demonstrating the high antitumor
efficacy of the novel XCLY-401759 mixture.
The strong paclitaxel-like antitumor efficacy of
XCLY-401759 is further shown in correlation data generated by
the NC1, as summarized below in Table 6:
i ~ I
CA 02210924 2002-09-06
- 42 -
TABLE 6
NCI
COhD?ARB-CORK-GI50 OOM (BV)
XCLY-401759/LCONC-4.
CORR.
NSC LCONC (MAX COBPF. (N) C1~M-NAi48
X)
1) 125973 -4.60 21 0.825 60 PACLITAXEL
2) 999991 0.00 1 O.All 10 t4DR RHOD30
3) 49842 -5.60 127 0.755 60 VINBLASTINE
SULFATE
4) 3053 -6.60 71 0.713 60 ACTINOMYCIN
D
5) 328426 -5.60 19 0.699 60 PHYLLANTHOSIDE
6) 337766 -3.60 10 0.686 60 BISANTRENE
HCL
7) 330500 -3.30 12 0.663 59 MACHECIN II
158) 165563 -3.70 14 0.618 60 HRUCEANTIN
9) 58514 -4.00 8 0.604 60 CHROMOMYCIN
A3
10) 267469 -3.70 13 0.590 60 DEOXY-
DOXORUHICIN
11) 83265 -3.90 15 0.586 60 S-TRITYL-L-
2 CYSTEINE
0
NCI
COMPARB-CORR-TCiI
XCLY-401759/LCONC-4.OOM (BV)
2 CORK .
5
NSC LCONC (MAX COSPF. (N) (~I-l~Dll~
X)
1) 125973 -4.60 20 0.830 59 PACLITAXEL
2) 49842 -5.60 128 0.727 59 VINBLASTINE
3 SULFATE
O
3) 332598 -9.00 9 0.605 59 RHIZOXIN
4) 153858 -4.00 15 0.598 59 MAYTANSINE
5) 67574 '3.00 62 0.527 59 VINCRISTINE
SULFATE
3 6) 330500 -3.30 12 0.501 59 MACBECIN II
S
7) 328426 -5.60 19 0.493 59 PHYLLANTHOSIDE
8) 83265 -3.90 15 0.484 59 S-TRITYL-L-
CYSTEINE
9) 325014 -3.65 11 0.451 59 BACTOBOLIN
4 10) 79037 -3.30 58 0.430 59 CCNU
0
i1) 349156 -3.65 11 0.422 59 PANCRATIASTATIN
*NSC-Test
number
LCONC-Log
of the
highest
concentration
tested
45 MARX-Total tests
number
of
COEFF.-Pearson lation coefficient
corre
CORR.-
(N)-Total cell lines
number
of
See Paul1 NCI (1989) 81:1088-1092
et
al.,
J.
CA 02210924 1997-07-21
- 43 -
5.2 IN VITRO STUDIES (SOUTf~RN RESEARCH INSTITUTE)
Additional in vitro studies were performed by the
Southern Research Institute, Birmingham, Alabama, an
independent research group, of the biological anti-cellular
5 activity of XCLY-401759 on four human tumor lines, MX-1
(breast carcinoma), RXF-393 (renal cell carcinoma), NCI-
H522 (lung adenocarcinoma) and OVCAR-3 (ovarian carcinoma).
In these studies, the XCLY-401759 analog was shown to yield
a range of activity comparable to paclitaxel.
l0 This testing was conducted using the
aforementioned human tumor cell lines employing standard
tissue culture techniques with semi-automated dye
conversion assays. Selection of the human cell lines for
testing was based at least in part on the following
15 criteria: (1) histogenesis of clinical import, (2)
adequate growth characteristics, and (3) the Institute's
experience with particular cell lines. The materials,
methods and results of this study follow.
5.2.1 Materials and Methods
20 5.2.1.1 Cell culture.
In the Southern Research Institute Study, human
cell lines were propagated under sterile conditions in RPMI
1640 (Hyclone) with 10% fetal bovine serum (Sigma
Chemical), 2 mM L-glutamine, and sodium bicarbonate
25 (complete medium) and incubated at 37°C in HEPA-filtered
Sterilcult COZ tissue culture incubators (Forma) with 5% COZ
and 95% humidity. The cell lines were subcultured weekly
to bi-weekly and used in experiments. All lines were
screened for mycoplasma contamination using GeneProbe""
30 (Fischer) and positive cultures were cured of contaminants
over three passages using constant treatment with BM-
Cyclin"" antibiotic combination (Boehringer Mannheim). Only
lines confirmed as mycoplasma free were used in testing
compounds for anticellular activity.
I II I
CA 02210924 2002-09-06
- 44
5.2.1.2 Anticellular activity experimental design.
For all experiments, cells were harvested and
pelleted to remove the medium and then suspended in fresh
complete medium. Samples were taken to determine cell
density. The cell count was determined with a Coulter
Model Z1"cell counter and viability was measured with
propidium iodide staining followed by analysis on a Coulter
EPICS Elite'Flow cytometer. The cell samples were adjusted
with complete medium to a density of 5 x 10' cells/ml.
Tissue culture cluster plates (96 well, cat No. 3595
Costar) were seeded with 100 u1 cells (5 x 103) and
incubated as described.
On the day of treatment analog XCLY-401759 was
dissolved in 100% ethanol, and then serially diluted in
medium. The 0 dose control was mock treated with medium.
The appropriate wells (columns of e) were treated with 5
concentration levels (10-°, 10-5, 10-6, 10'', and 10-8 M) . The
highest dose of initial vehicle (ethanol in media) was <
0.2% ethanol. A vehicle control was prepared at 0.2% to
2o determine the effects of vehicle on the cell lines.
Paclitaxel supplied by XECHEM, Inc., New Brunswick, New
Jersey, was dissolved in DMSO, serially diluted in medium
and then added to the wells to achieve doses of 1 x 108 and
1 x 10-9 M. Each cluster plate contained a cell control (8
wells, mock-treated with complete medium), a medium control
(7 wells with medium used to substract out signal generated
by medium conditions) and an air blank (1 well, for
calibrating the plate reader). Once dosing was completed,
the plates were stacked and wrapped in plastic film to
reduce evaporation and incubated as described. Replicate
sets of cluster plates had either 1 hour or 72 hour drug
exposure. For the appropriate drug exposure, the plates
were aseptically blotted on sterile towels and gently
washed three times with medium. The samples were then fed
with fresh medium, and the plates were wrapped in plastic
wrap. The plates of both exposure sets were incubated to
day 7 and then processed to analyze for anticellular
activity using the sulforhodamine B (SRB) procedure.
~i~ ,
CA 02210924 2002-09-06
- 45
5.2.1.3 Results
In the 1 hour exposure XCLY-401759 concentration
dependent activity was demonstrated in all the tested cell
lines. OVCAR-3 ovarian and NCI-H522 lung cell lines were
5 the most sensitive to XCLY-401759. Paclitaxel activity was
minimal at the two concentrations tested for MX-l, RXF 393
and OVACAR-3 tumor cell lines, with NCI-H522 showing
sensitivity to paclitaxel. All cell lines showed increased
sensitivity to both XCLY-401759 and paclitaxel when the
10 exposure time was increased to 72 hours. MX-1 was
relatively less sensitive than other lines to paclitaxel
and XCLY-401759.
In summary, according to the Southern Research
institute's Study, XCLY-401759 yielded a range of
15 anticellular activity comparable to paclitaxel in four
human tumor cell lines of tested various neoplastic disease
criginans.
The results are summarized below in Tables 7 and 8.
20 TABLE 7
SODTfH;RN RESEARCH INSTITUTE
TREATMENT DAY 1 POST PLATING-PLATES WASHED
1 HIt AFTER Rx; PLATES READ ON DAY 7
2 5 t INHIBITION
TRBATI4HZiT CELL LINECELLS PLATEDS.OS+03CSLIS/WSLL)
(
AG~1T 1M) R7CP 393 h~C-1 OVG\R-3 NCI-H522
XCLY-401759 1.0E-08 2.5 3.2 23.1 7.9
1. OE-07 16.0 3.7 81.2 24.8
30 1. OE-06 23.8 0.0 97.2 95.2
1. OE-05 42.9 1.7 98.1 99.4
1. OE-04 38.1 42.7 98.3 99.5
VEHICLE CONTROL 0.0 0.8 7.0 4.5
PACLITAXEL 1.0E-09 3.7 1.6 1.2 8.3
35 1. OE-08 13.8 4.0 7.1 35.1
~i
CA 02210924 2002-09-06
- 46
TABLE 8
SOUTHERN RESEARCH INSTITUTE
TREATh~NT DAY 1 POST PLATING-PLATES WASHED
72 HRS AFTER Rx; PLATES READ ON DAY 7
t INHIBITION
TTtSATMHrIT CBLL LINE HLZS PLATBn5.08+03CBLLS/WBLL)
(C
AG~1T (M) RXF 393 MR-1 OVCAR -3 HCI-H522
XCLY-401759 1. OE-08 64.8 29.4 98.7 97.2
1. OE-07 80.7 45.4 99.1 98.6
1. OE-06 85.1 76.5 99.2 98.4
1. OE-05 81.6 75.4 98.8 98.3
1. OE-04 100.0 98.3 100.0 100.0
VEHICLE CONTROL 4.4 2.3 0.0 0.0
PACLITAXEL 1. OE-09 41.5 10.6 98.1 96.1
1. OE-08 73.3 41.1 99.3 98.7
5.3 IN VIVO STUDIES
In vivo hollow fiber assays were performed by the
NCI Developmental Therapeutics Program on the anti-cellular
20 efficacy of the inventive XCLY-401759 analog on several
neoplastic tumor cell lines.
This testing was performed by the Biological
Testing Branch of the Developmental Therapeutics Program.
In these assays, human tumor cells as indicated were
25 cultivated in polyvinylidene fluoride (PVDF) hollow fibers,
and a sample of each cell line implanted into each of two
physiologic compartments (intraperitoneal and subcutaneous)
in mice. Each test mouse received a total of six fibers (3
intraperitoneally and 3 subcutaneously) representing 3
30 distinct cancer cell lines.
Three mice were treated with potential antitumor
compounds at each of 2 test doses by the intraperitoneal
route using a QD x 4 treatment schedule. Vehicle controls
consisted of 6 mice receiving the compound diluent only.
35 The fiber cultures were collected on the day following the
.last day of treatment.
i
CA 02210924 2002-09-06
47 -
To determine antitumor efficacy, the viable cell
mass was determined for each of the cell lines using a
formazan dye (MTT) conversion assay. From this, the % T/C
was calculated using the average optical density of the
5 compound treated samples divided by the average optical
density of the vehicle controls. The net increase in cell
mass was determined for each sample.
The XCLY-401759 diastereomeric mixture/compound
was tested against a minimum of 12 human cancer cell lines,
amounting to a total of 4 experiments as each experiment
contains 3 cell lines. The data are reported as °sT/C for
each of the 2 compound doses against each of the cell lines ,
with separate values calculated for the intraperitoneal and
subcutaneous samples.
15 The results of this in vivo assay are summarized
below in Tables 9-12.
CA 02210924 2002-09-06
O1 1J
1J
No ~ 3 3
U o o~
G4U7 ~--~ ?~
?.
v~n ro ro
ro
0 O w
.L~
.L7
CL ~
~
r-, a~ E E >
N
0o ro
00 ~~ v
0o w
n n b
E E
U ~ ~ m
U
1J >LL N O O O
O
v OH w ~o .. U
..
v
r1
r-1
> >
U o ~r
z w Uow ro
E m ncn ri
O U ow ~n -n t0
n
d~
i N I-1 UI
w c~
u,
H ~ ~n
~ G E
o .. x x ~o
..
'w . w w m
O ~ ~
'' H tx N N c'~1 Gl~
b v~ ' .R
~
O O 0 w --
--
x~n zw o
N ~~
O O JJ
x
o0 0
w
ov
U O O ?.
r-I -.1 r~ ~ a0 t~
rn
z~
..
o
x
~.
..
aA
b a v u~ ro
v
w ,-~ . ~ c
.
r'' w a ~ ~ .~, sL
-.~
U ro ~--~ v
~
x a~ x ~ ~a v
x
b
U O U Ll
fa
~n H ~n a c c
a
z HH
w
..
..
H ~ .u
w c~
a )
xHH
W o 0 3
cx o 0
Crr E-~ Ci. U1
x N v o o x ~
o u~ c m n o
o0
roro c
o, ~ ~ . . t~ v
u~ ~ Cn v N ov E
r~. .~ x u~
u~
x w ~n ~ o o w
~
~ a~ A a x v
rn
w E E V v C1~
..
..
O a o o ~ ~ O v
... z . o o w
~ . . n n
x ~n o v~ . . v~ -~
o
xw ono w H .c
wcn r~~~ a M~ z H
U W
H
a as
o w o
~
c~z > ~c~ U
r~~
i
CA 02210924 2002-09-06
U ~a
v a~
m
3
3
rn 'U
"d
i Ch N O
l0 O
H lD .L~ 'b
(7 .L~
a
U E
E
> CJ1 r0
01
O o
o
U rwn r-~
H
G7 av \ 27
av \
U
E ~,
E
N
b
x a, a
~
1J t!1H (~ O U7
01 O
3 0 G
O N O
fn f-1 r-I U
r-I
C~
~ U o > ~n
~ >
'~ ~ ~ O cn
Z N U n
~
r'' z -~-~ b
U
-rl H
H
~
' ~r \ H lf1 (n
tl1
~ w ~ ~
~
U
3 v
3
x a a H o
0
N ~
~
~ . E
O .
.. H C tC
.. C
W W ~ i~
U7 ~
U x o -- ~c
~ --
Hx o a~ a
ro ra N f~1 __
. ('~
O O O ~o r1
~ dP
xcn zw ~n~n o
~
W
o. ,.. a .
a .
~ x o0
E-~ ~ w U
0
U c0 ~.
ao
. r~ y~
.,.~<n
~.,~
o e
~ ~
,o v
z v
a~
v
x ~ ~r ~ tr
~
.. ~,, ~,, ..
..
A a s
a~ ~
b v
a~ c ~a
~~ ~
. ,~ . .r.,
. .r.,
.r,,
z o ~ ~ v
~ o
A' ~
H v x cn U
x cn
U v U U
G1 C~ ~ c
~ 0
w ~n a
a
U
F, ..
..
x z
__
C2.'H O -.-i
H O
0 3
0
~
W o Cs. m
o
x v v ~n x
o
E-~cn m H
~
w o 0 0
x b w ..
\ \ vv ~ v
o ~ Cn u~ o~ E
m
.x .x O m .a
0
U1 \ C~ w Yr
\ La
~ rn -- x v
a, -
w E a
E
G.
x tr. ~ 0 0
0
_. o o w
U . n
. n
.. r~ o v~
.. o
o o o w
~n
z o~ ~ a ~,~ ~ H
U N
~ W ~
xW O ~ O
f W ~ C7 ~ > C.~ U
Z C7
r~
i
CA 02210924 2002-09-06
O
U M o
~ m
n 3 3
b
(3, N O O
OD
OH CD 01 (a
,Q
N -ri
E E
o tT
~
3 0 00 >
cnU o~ ~ ~,
~-..~
u7 n \ \
av
v
E E
v
b
.C MCL e~
O
JJ NH ~ N O O U1
..
..
O CG 0
U7 ~I ~ O .-~ U
r-I
U
b ~U ~ ~ > > u7
G7v7 n
2 v g . .
z .n.n .d
O U V C C
.r~ H H (d
U GL ~-1
N
a
\ H ("~ lD fn
p O 0
~C
~
N N ~ E
~
o ..
.
.. x c ~ ~a
' ..
~'a . w .w ~n D 5
U HO S~ -, ~C
.--
ro ~' z~ '
~
c M M
n .
0 0 0 -~
x~n zw u,~n o
H o o ~r
o z o0
0
N ~
,i w U
O ~ O O
U co >,
m
..,-I ~
l1
0 ~ P~~~
a~
v
a~
a~
I
M M ~ ~ o'
'
x
.. ~, ~, ..
..
'~ a A
~ ~
v c ~ ro
~, _
~' _
w a ~ w ,~ a~
~
z ~ ~o v
~c
ro
H a~ x cn U
~c cn
V v U
!~ L1
w ~n a
a
U
..
..
D z
O W ~~ W .-.
w ....
(n "E (x H O O r1
H
0 0 3
w o o fs, ~n
x N N ui x
o
H ~ ~ ~~
w o 0 0
x ~ b ..
\ \ v u, ~ v
0 0~ O~ m u~ a~ E
,x x o 0
cn \ o ca w
\
o, ~ rn rn -- x a~
--
u~ .~ E C1
E
w c ~~ ~ x
-.. xCs. 0o 0 o v
0 o w
U . . n n u~
.. u~ o cn . . u~
.. o
o o~ o w
z . a~, ,~ a M~
U
E" H E
'
W
W
C C7 ~ > C~ U
2 t7
M
CA 02210924 2002-09-06
U ~' C
Cn 41 O~ 1.!
1J
3 3
w ao ~ O O
H Lll .D 'b
.Ca
H
E E
N O
O O O
U o H .-,
,-~
c~ ~ n
E E
v
_ H ,..i
GL N C' r1
1J H l0 l0 O O
3 N ..
..
0 ~c p
U1 1.a ~ O a0 ~ r-1 U
' ~ ~
'O U
~ U
~ ~ a u~
n
z v
o ~ W~ b
a, v >~ c
c
_ H H
U LL lI1 Q1
~ H I~ ~ ~'
U
x ~ E. c ~ S
..~ -I
N O O 1J
ja N ~ G v
O
x
W w ~
U H O ra
~
l7 z '
a ~
-y m r r~ r, ....~
rs nu
x
~ c z e
n
i a ~"r o 0
z
~ o0
0
w
U
0 N O O
U aD >,,
a>
~~ r, r'~
~
z
,,~ v v
~
'~
x ~ ~ o,
.. .. v
..
c~ a v v ~a
ro
v ~~ ro
H
w a ~
c ~ ~ c~
' ~
ro
a ~x x c v
ro n
w ~ a a
~
-
.
U
G
.. .,.,
..
o w ~I a, w ....
~.
U7 ~ f1' H O O m-i
H
0 o g
W o o c=.,m
x v v u,
o
H u1 u7 H H
w o 0 0
x b b
v v ~n v
o tr~ Cn ~n av E
m
x x o o m
w w o a w s~
u; ~ E VV x v
E
. '
~
w G ~ H ~ k
__ x ~" ~ 0 0 o v
0 o w
v , . n n
~n o o cn
a
E-' H
' E
~ Z
W r~ ~ ~ C7 U
I C7 C7
CA 02210924 1997-07-21
- 52 -
EXAMPLE 6
PREPARATION OF 2",3"-DICHLOROCEPHAhOMANNINE
DIASTEREOMERS AND BIOLOGICAL ACTIVITY STUDIES
5 6.1 Raw Materials
Batches of crude plant extracts from Taxus
yunnanensis or from Taxus wallachiana containing
approximately 15-40% cephalomannine, approximately 50-70%
10 paclitaxel, and approximately 20-35% other taxane/non
taxane components were obtained from The People's
Republic of China. Chlorine gas was obtained from ,
Matheson Ltd. Silica gel used was ICN Silitech, 32-63
um, 60 A, ICN Biomedicals, Inc., Aurora, OH. All
15 solvents used were either HPLC or ACS grade and were
obtained from Spectrum Chemical Mfg. Corp. Purified
water used was deionized in-house.
6.2 Chlorination of Crude Plant
20 Extract in Oxidized Chloroform
6.2.1 Preparation of Oxidized Chloroform
Chlorine (3.12 g) was added dropwise to
25 chloroform (4 1) in order to neutralize a stabilizer,
amylene, present in the commercially available solvent.
The solution was mixed vigorously and left standing at
room.temperature overnight, and then washed once with
1.5% sodium sulfite solution (1.0 1), and twice with
30 water (2x1.0 1). Hydrogen peroxide solution (3%, 10 ml)
was then added, mixed vigorously, and allowed to stand
for 3-5 days. Chlorine content in the solvent was
determined by volumetric analysis. Next, to the solvent
sample (5 ml) was added 1.0 N HC1 (10 ml) and water (50
35 ml. To this mixture was then added KI (2 g), mixed well
to dissolve, and the resulting dark brown solution
titrated with 0.1 N sodium thiosulfate solution. As the
color of solution turned light brown, 3-4 drops of starch
indicator solution (0.5%, USP) were added. The dark blue
CA 02210924 1997-07-21
- 53 -
- purple solution was further titrated until the solution
turned colorless. The volume of sodium thiosulfate
solution used to arrive at the end point was noted and
chlorine content calculated. The desired chlorine
5 content was in the range of 0.01 - 0.1%. The solvent was
dried with anhydrous sodium sulfate (100 g) and used for
the following chlorination reaction.
6.2.2 Chlorination
10 Crude plant extract (5.0 g, 28.8% cephaloman-
nine, 62.2% paclitaxel) was dissolved in oxidized chloro-
form (1 1) in a 31 flask cooled to 4°C using an ice bath.
HPLC analysis of the mixture after 1 hour showed a
paclitaxel to cephalomannine ratio of 8:1. The reaction
15 mixture was then stirred at 15°C for 9 hrs. HPLC analysis
of the reaction mixture at this point showed a paclitaxel
to cephalomannine ratio of 19:1. The reaction mixture 5
ml sample washed with 5 ml deionized water had a pH of
about 2Ø The mixture was then washed with 500 ml 1.0%
20 aqueous sodium sulfite solution, and the pH of the
aqueous layer was 7.5. This was followed by two washes
with water (2x500 ml). The pH values of first and second
water washes were 7.0 and 6.5, respectively. The
combined aqueous layer was re-extracted with 150 ml
25 chloroform. The organic layers were combined, dried with
anhydrous sodium sulfate (85 g), and evaporated to
dryness. The solid residue (5.85 g) was purified by
chromatography. LCMS analysis of the chlorinated
material indicated formation a diastereomer mixture of
30 dichlorocephalomannine as the product of reaction along
with paclitaxel present in the starting material.
CA 02210924 1997-07-21
- 54 -
6.3 Chromatographic Purification
of Chlorinated Material
The chlorinated material (5.85 g) was
5 chromatographically purified using a column (4.1 cm i.d.,
62 cm long) packed with silica gel (300 g) by the slurry
method using 10% acetone in 1,2-dichloroethane. The
sample was dissolved in 10% acetone in 1,2-
dichloroethane. Following the first two 700 and 350 ml
10 fractions, all subsequent fractions were limited to 50 ml
each. The fractions were analyzed by TLC (TLC plates
were developed with 20% acetone in 1,2-dichloroethane,
detected with 1% vanillin in 50/50 sulfuric acid-
methanol). Dichlorocephalomannines eluted in fractions
15 8-13 and yielded 1.6 g solids (-90%) following
evaporation of solvents. This material was finally
purified by semi-preparative HPLC.
6.4 Chlorination of Crude Plant
20 Extract in 1,2-Dichloroethane
6.4.1 Preparation of Chlorine
Solution in 1,2-Dichoroethane
25 A solution of chlorine in 1,2-dichloroethane
was prepared by slow bubbling of chlorine into 1,2-
dichloroethane (1 1) precooled to 0 - 4°C using an ice
bath. The bubbling was continued for several min.
(approx. 10 min.) until the desired concentration of
30 chlorine in 1,2-dichloroethane was achieved. Samples of
the solvent were withdrawn periodically and analyzed for
dissolved chlorine content as follows: To the solvent
sample (5 ml) in a 250 ml erlenmeyer flask were added 1.0
N HC1 (10 ml) and water (50 ml). To this mixture was
35 added KI (2 g), mixed well to dissolve, and the dark
brown solution was titrated with 0.1 N sodium thiosulfate
solution. As the color of solution turned light brown,
3-4 drops of starch indicator solution (0.5%, USP) were
added. The dark blue - purple solution was further
CA 02210924 1997-07-21
- 55 -
titrated until the solution turned colorless. The volume
of sodium thiosulfate solution used to arrive at the end
point was noted and chlorine content was calculated. The
desired chlorine content was in the range of 0.01-0.1%.
5
6.4.2 Chlorination
Crude plant extract (5.0 g) dissolved in 1,2-
dichloroethane (200 ml) was cooled to -4°C and added
dropwise to the stirred solution of chlorine (0.06%) in
10 1,2-dichloroethane (1250 ml) cooled to 4°C by using an ice
bath. Following complete addition, the mixture was
stirred at 4°C for 1 hr and a sample was analyzed by HPLC.
HPLC analysis indicated that the cephalomannine peak was
nearly completely eliminated. The mixture was washed
15 with 1.0% sodium sulfite solution (1 1) and water (2 x 1
1). The pH values of the aqueous layers were as follows:
sodium sulfite wash, 7.5-8.0; first water wash, 6.0-6.5;
second water wash, 5.5. The aqueous layers were
extracted with 1,2-dichloroethane (200 ml). The organic
20 layers were combined, dried with anhydrous sodium sulfate
(50 g) and evaporated using a rotary evaporator at 40°C.
The residual solids were dried in vacuum oven at 40°C for
2 hrs to yield 5.3 g chlorinated material. HPLC analysis
of this material showed dichlorocephalomannine as the
25 product of the reaction together with paclitaxel present
in the starting crude plant extract.
6.5 Separation of Chlorinated Material
From Paclitaxel by Crystalization
30
The chlorinated product mixture from 6.4.2
(5.30 g) was dissolved in acetone (50 ml) in a 250 ml
Erlenmeyer flask. To this solution was added hexanes (65
ml), mixed well, and let stand at room temperature until
35 crystallization began to occur. The flask was then
stored at 4°C for 60 hrs. The crystals were filtered,
washed with cold 20% acetone in hexanes, and dried in
iv
CA 02210924 2002-09-06
- 56 -
vacuum oven at 40'C for 3.5 hrs to yield 3.10 g paclitaxel
(- 95%, crystals I). The combined filtrate and washings
were evaporated, and the residual solids dried in a
vacuum oven at 40°C for 2 hrs to yield 1.96 g mother
5 liquor material (mother liquor I). The crystals I (3.10
g) were next dissolved in acetone (32 ml). To this
solution was added hexanes (40 ml) and the mixture stored
at room temperature,for 5 hrs and then at 4°C overnight.
The crystals were filtered, washed with 20% acetone in
10 hexanes, and dried in vacuum oven at 40°C for 3 hrs to
yield 2.49g paclitaxel, 98.5% (crystals II). The
filtrate and washings were combined and evaporated. The
residual solids were dried in a vacuum oven at 40°C for 2
hrs to yield 0.65 g mother liquor material (mother liquor
15 II). The crystals II (2.49 g) were again dissolved in
warm acetone (25 ml). To the solution was added hexanes
(25 ml) and the mixture stored at room temperature for 5
hrs and then at 4°C overnight. The crystals were
filtered, washed with 20% acetone in hexanes, and dried
20 in a vacuum oven at 40°C to yield 2.01g paclitaxel (99.5%,
crystals III). The filtrate and washings were combined
and evaporated. The residual solids were dried in a
vacuum oven at 40'C for 2 hours to yield 0.478 mother
liquor material (mother liquor III). The mother liquors
25 I, II, and III containing dichlorocephalomannines were
then pooled and further separated by semi-preparative
HPLC.
6.6 Final Purification of 2", 3" -
30 Dichlorocephalomannine and
2",3"-Dichloro-'7-epi-cephalomannine
Diastereomers
The final purification of dichlorocephaloman-
35 nine and 7-epi-dichlorocephalomannine diastereomers from
other impurities was accomplished by semi-preparative
HPLC (Waters Deltaprep" 3000) using a Waters Deltapak° C18
column, 100 P. l9mm x 30 cm with 45% acetonitrile in water
I
i I ~ i
CA 02210924 2002-09-06
- 57 -
as the mobile phase at the flow rate of 15 ml/min. Peak
elution was monitored using a W detector set at 227 nm.
P~~rions of 200 mg material dissolved in methanol (2 ml)
were injected. Elution ~~i ciichl~rocephalor,4~nnine
3iastereomer I peaked approximately at 86 min. and
diastereomer II at 98 min. Likewise, the dichloro-
cephalomannine diastereomer III peaked at approximately
118 min and the corresponding diastereomer IV peaked at
124 min respectively. Fractions collected from repeated
injections were pooled and evaporated at 40°C under
reduced pressure to remove the organic solvent. The ,
crystallized solids were filtered, washed with water, and
dried in vacuum oven at 40°C to yield pure dichloro-
cephalomannine.
15 diastereomers. The dichlorocephalomannine diastereomer I
isolated in this manner was associated with a- contaminant
and was repurified by collecting smaller fractions during
peak elution following the described HPLC procedure.
The preparation, separation and structures of
the obtained diastereomeric dichloro compounds,
(I) (2"R, 3"S)-dichlorocephalomannine, (DiCl-I)
(2"R, 3"S)-dichloro-7-epi-cephalomannine;
(II) (2"S, 3"R)-dichlorocephalomannine, (DiCl-II)
(2"S, 3"R)-dichloro-7-epi-cephalomannine;
(III) (2"R, 3"R)-dichloro-cephalomannine, (DiCl-III)
(2"R, 3"R)-dichloro-7-epi-cephalomannine;
(IV) (2"S, 3"S)-dichloro-cephalomannine, (DiCl-IV)
(2"S, 3"S)-dichloro-7-epi-cephalomannine are
shown
in scheme VII:
i.
CA 02210924 2002-09-06
- 58 -
S =heme VI I ,
"~o o .~0 0
- » .
a » . , r r ° " ' , ,
»
II 11 1 1 r
r , ~ r r r a ~ ,
O Aa0 s . " g p AcD
H .' H
own o~n
Paditascl Cep6aiomanoiae
7 . tFi . c~,Y,manntoe
' <CC1J
((~~
(CIi~
<C~H,O~
,_
~.p o ..o o" ~ »
p ° O ° ° ., ~ ,
a " ~ ~ , r, » , s ~ s r
" , a r . ,
w
r ~ ~ ° ~ ,
H ~ , ~ p ~ ~o
ooh own ° n
Paditasd I"r 3" -dfelisro ~ aptrabaVnei~e Z", 3" ~ dkhloro ~ 7. cpi .
apfaalamaeoiae
Separation
Analogues (2"R , 3"S) - did~lorocepdalomannine Anaiogue I T(I"S , 3"R) -
diehMrvcephalomannine
- (2"R,3"S)-dichloro-?-epi-cephalomannine - (2"S,3"R)-dichloro-7-epi-
cephalomannine
Analogue (2"R , 3"R ) dic6loro .cephalomanninc Analogue~~(Z"S , 3"S ) dichloro-
:-cephalomannioe
- (2"R,3"R)-dichloro-7-epi-cephalomannine - (2"S,3"S)-dichloro-7-ep1-
cephalomannine
Paclitaael Aaalogues (Chlorinated)
~i i
CA 02210924 2002-09-06
_ 59 _
Analytical characterization of these
diastereomers follows.
Fig. 14 shows TLC separation of 2",3"-
dichlorocephalomannine and 2',3'-dichloro-7-epi-cephalomannine
stereoisomers (DiCl-I-DiCl-IV). A key to Fig. 14 is set forth
below in Table 13.
TABLE 13
Lane No. Stereoisomer
1 DiCl-I
2 DiCl-II
Paclitaxel
3 DiCl-III
4 DiCl-IV
20
Plate: silica gel 60 F254' (Merck #5554)
Solvent System: a) 10% CH30H in 1,2-dichloroethane
b) hexane/chloroform/EtOAc/CH,OH
20:60:15:5
Reagent: a) W light
b) vanilin/HzSOa in methanol
FIG. 15 is an HPLC chromatogram of a mixture of
the dichlocephalomannine and dichloro-7-epi-
cephalomannine diastereomers of this invention, with
peaks identified below in Table 14.
TABLE 14
Peak No. ~tereoisomer
I DiCl-I
II DiCl-II
III DiCl-III
IV DiCl-IV
f
i I i
CA 02210924 2002-09-06
- 60 -
Equipment and conditions employed in generating
this chromatogram are as follows:
column: ES' Industries FSP' (pentafluorophenyl); 4,6mm
ID x 250 mm; 5 um; 60 A
solvent system: water/acetonitrile/methanol, 41:39:20
flow rate: 0.50 ml/min.; isocratic
detector: waters 990="'photodiode Array Detector, monitored
at 227 nm
injection volume: 20 ~tl .
FIG. 16 shows a comparison of the W spectra for
diastereomers DiCl-I, DiCl-II; DiCl-III and DiCl-IV in CH30H.
Spectra results are summarized ~~slow in Table 15.
TABLE 15
Peak No. Stereoisomer Amax, nm
I DiCl-I 226.6 14,813
II DiCl-II 227.2 14,990
III DiCl-III 228.2 17,252
TZ~ DiCl-IV 229.4 1n,694
FIG. 17 shows a comparison of the IR spectra for
diastereomers DiCl-I, DiCl-II, DiCl-III and DiCl-IV, which are
summarized below in Table 16.
TABLE 16
Band- cm-1 Functional Groys
3500,1105,1070 tert, and sec. OH
3420,1670,1580 -CONH-
3110,3060,1605 mono sub. aromatic rings
1505,770,710
2960,2915,2870 -CH3-; -CHZ-; -CH-groups
1465,1370 tin aliphatic or cyclic
compounds)
--- 3020,1670,1310 double bonds
980
'., i ~~. ~ ~i
CA 02210924 2002-09-06
- 61 -
1730,1270 aromatic esters
1715,1240 >=0 groups
1730,1180 acetates
855 oxetane rings
FIG. 18 is the NMR spectrum diastereomer
1H for
DiCl-I.
FIG. 19 is the NMR spectrum diastereomer
1H for
DiCl-II.
FIG. 20 is the NMR spectrum diastereomer
'H for
DiCl-III.
FIG. 21 is the NMR spectrum diastereomer
1H for
DiCl-IV.
FIG. 22 shows 13C-NMR spectrum
the for diastereomers
DiCl-I, II, III The 'H NMR and C-NMR spectra
and " for
IV.
each diaste reomer follows:
is
summarized
below
as
DILL-I 'H-Nl~t in ~CL,
(300
I~iz
in ppm;
side
chain
an d some ortant Drotons ne ~~
imfl o
Chemical Shift Ass ianm nrs
(~~m)
2. 54 (m,1H) - (H - 6a)
1.92 (t,1H) -(H - 6b)
2 .32 (m,2H) - (H - 14a)
2.32 (m,2H) -(H - 14b)
4.58 (q,1H) -(>CH - C1 - C3")
1.55 (d,3H) - (HC - CH3 - C,")
CL
i
i
1 .70 (s, 3H) ( II C CH, - CS" )
0 CL
CA 02210924 1997-07-21
- 62 -
DILL-I 13C-NMR in CDCL3
(300 Ngiz in ppm; side chain
and some important carbons onl
S Chemical Shift tppm) Assignments
170.2 -(C - 1' ; C = O)
73.1 -(C - 2')
10
55.0 -(C - 3')
172.0 -(C - 1') C = 0)
15 70.8 -(C - 2")
58.7 -(C - 3")
21.8 -(C - 4")
20
27.5 -(C - 5")
203.6 -(C - 9; C = O)
CA 02210924 1997-07-21
- 63 -
DICL-II 1H-NMR in CDCL3
(300 I~iz in ppm; side chain
and some important protons only)
Chemical Shift (ppm) Assignments
2 . 56 (m,1H) - (H - 6a)
1.94 (t,1H) -(H - 6b)
2.34 (m,2H) -(H - 14a)
2.34 (m,2H) -(H - 14b)
4 . 58 (q,1H) - ( >CH - C1 - C3"
)
1 . 55 (d,3H) - (HC - CH3 - C4"
)
i
i
Cl i
i
1.70 (s,3H) - (-~~ - C - CH3 -
CS
i
i
0 C1
DILL-II13C-NMR
(300 Ngiz in ppm; side chain
and some important carbons only)
Chemical Shift (ppm) Assignments
170.2 -(C - 1' ; C =
O)
72.6 -(C - 2')
55.0 -(C - 3')
172.6 -(C - 1") C = O)
70.6 -(C - 2")
58.7 -(C - 3")
21.8 -(C - 4")
27.7 -(C - 5")
203.5 -(C - 9; C =
O)
CA 02210924 1997-07-21
- 64 -
DILL-III 1H-NMR in CDCL3
(300 I~iz in ppm; side chain
and some importantprotons only)
S Chemical Shift (ppm) Assignments
2.35 (m, 2H) - (H - 6a)
2.35 (m, 2H) -(H - 6b)
10
2.54 (m, 1H) -(H - 14a)
2 . 35 (m, 1H) - (H - 14b)
15 4.50 (qt, 1H) -(>CH - C1 - C3")
1 . 52 (d, 3H) - (HC - CH3 - C4" )
i
C1
20
25
i
i
1.28 (s, 3H) - (-CII- C - CH3 - CS")
i
i
O C1
DILL-III13C-NMR
(300 MHz in ppm; side chain
and some important carbons onlv)
30
Chemical Shift (ppm) Assignments
170.2 -(C - 1' ; C = O)
35 73.0 -(C - 2')
54.8 -(C - 3')
172.2 -(C - 1") C = O)
40
62.7 -(C - 2")
55.3 -(C - 3")
45 21.6 -(C - 4")
29.3 -(C - 5")
203 . 5 - (C - 9; C = 0)
50
CA 02210924 1997-07-21
- 65 -
DICL-IV lA-NMR in CDCL3
(300 NIfIz in ppm;side chain
and some importantprotons only)
Chemical Shift (ppm) Assicrnments
2.35 (m, 2H) -(H - 6a)
2.35 (m, 2H) - (H - 6b)
10
2 . 54 (m, 1H) - (H - 14a)
2.35 (m, 1H) -(H - 14b)
15 4.50 (q, 1H) -(>CH - C1 - C3")
1 . 52 (d, 3H) - (HC~ - CH3 - C4" )
-
C1
20
i
i
1 .28 (s, 3H) ( ~~ C CH3 - CS" )
i
i
O C1
25
DICL-IV1'C-NMR
(300 I~iz in ppm; chain
side
and some important
carbons only)
30 '
Chemical Shift (ppm) Assignments
170.2 -(C - 1' ; C = O)
35 72.9 -(C - 2')
53.9 -(C - 3')
172.2 -(C - 1") C = O)
40
62.5 -(C - 2")
55.0 -(C - 3")
45 21.7 -(C - 4")
29.3 -(C - 5".)
203.5 -(C - 9; C = O)
50
i ~ s
CA 02210924 2002-09-06
66 -
FIG. 23 is an EI mass spectrum of the DiCl-IV
diastereomer, which is the same fragmentation pattern for
diastereomers DiCl-I, DiCl-II and DiCl-III.
FIG. 24 is a FAB' mass spectrum of diastereomer DiCl-II,
which is the same spectrum for diastereomer DiCl-I, DiCl-III
and DiCl-IV. Data from Fig. 23 and Fig. 24 are summarized
below.
E1-MS; [M'] =902 568 [T]'; 550 [T - Hz0]';
508 [T-AcOH] +; 490 (T-AcOH-H20)';
(m/z, the main 480; 448 (T-2AcOH)+ or [T-BZOH]'; 386
fragments) (T-AcOH-BZOH]'326 (T-BZOH-
2AcOH]'; 308 (T-326-Hs0]'; 264 [832-T] +;
246 [264-H=O]'; 188; 148; 122 [BZOH)'; 105
(g=] '; 91 (C,H.,)': 83 (C,H,C=O) ; 77 (C6H5]';
57; 55; 43
DiCl-I, DiCl-II, DiCl-III and DiCl-IV (m/z, the main
fragments),
940 ( [M+K]') ; 924 ( (M+Na] ') ; 902 ( (M+1H]') ;
842 ( [M-60'] ) ; 832 ( [cephal) ) ; 824 ( [M-60-18)') ;
569 ( [T]') ; 551 ( [T-18]') ; 527 ( (T-43]') ;
509 ( (T-60]') ; 491 ( [T-60-18]') ; 449/448 ( [T-122]') ; 405
( [S-18] ) ;
387([T-60-122]'); 327([387-60]');
309 ( [327-18]') ; 264 ( (832-T]') ;
246 ( (264-18]') ; 218 ( (264-46]') ;
105 ( [C~HSCO] ') ; 91 ( [C,H,] ') ; 77 ( (C6H5] ') ;
I i
CA 02210924 2002-09-06
- 67 -
Physico-chemical properties of the
dichlorocephalomannine di~.~-ereomers ef this invention are
summarized in Table 17.
TABLE 17
Phvsico Chemical Properties of Chloro-Analorn~es of
Paclitaxel
Property DiCl-I Di-Cl-II DiCl-III DiCl-IV
Appearance white to White to White to White to
off-white off-white off-white '
crystals crystals crystals off-white
crystals
Melting 190-192'C 186-188C 178-182'C 160-162C
point
Molecular Cq5H53~14NC12Cq5H53~14NC12C45H53014NC1ZCq5H53O:qNCl~
(
i5 formula
Molecular 902.8 902.8 902.8 902.8
weight
(~) D -56 . 9' -45. 9 -38 . 8
IR*(cm-1) 3500, 1105,
1070; 3420,
1670, 1580;
3110, 3060,
1605, 1505,
770, 710;
2960, 2915,
2870, 1465,
1370; 3020,
1670, 1310,
980; 1730,
1270; 1715,
1240; 1730,
1180; 855;
760
W ~."ax; 226.6 nm; 227.2nm; 228.2 nm; 229.4 nm;
(e) 4813 14990 17252 14694
1
TLC** (Rt)
solvents
A; 0.41 0.43 0.46 0.49
g, 0.33 0.36 0.39 0.44
HPLC***
(RT)
condition
1; 38.50 min. 41.75 min. 48.29 min. 49.74 min.
condition
2; 37.75 min. 41.83 min. 45.98 48.01 min.
*The IR spectra of DiCl-I-IV are superimposable.
f i~ t
CA 02210924 2002-09-06
- 68 -
**Solvent System A: Methanol-1,2,-Dichloroethane-
(1:10).
Solvent System H: Hexane-Chloroform-Ethylacetate-
Methanol-(2:6:1.5:0.5?
*** Condition 1: Column: ES~ Industries FSP
(Pentafluorophenyl) 4.6 mm ID x 250 mm, 5 um particle
size, 60~ pore size; mobile ~has~ - water - acetonitrile
- methanol - (41:39:20); flow rate 0.50 ml/min;
seoaratiQn mode - isocratic; detgstor - Waters 990"
Photodiode Array Detector; elution monitored at 227 nm;
infection volume - 20 u1.
Condition 2: C : Phenomenex 4.6 mm ID x 250 mm,
S ~m particle size, 80 pore size; mobile phase - water -
acetonitrile - methanol - (45:40:15); flow rate - 0.50
ml/min; separation mode - isocratic; ~etesaor - Waters
490 programmable multiwavelength detector, elution
monitored at 227 nm; infection volume - 80 u1 total
mixture.
EXAMPLE 7
In Vitro NCI Studies Showing
Antitumor Efficacy of (2"R, 3"S) -
aad !2"S,3"R)-Dichloro-
Cephalomannine Diastereomers.
In this NCI study, isolated and purified
(2"R,3"S) and (2"S,3"R) diastereomers of dichloro-
cephalomannine are shown to exhibit strong paclitaxel-
like antitumor efficacy in vitro in the NCI's sixty human
tumor cell line screen.
7.1 Discussion of Results
The results of the NCI in vitro study are
summarized below. Tables 18A-18F show data sheets of in vitro
testing results of diastereomer (2"R, 3"S)-
dichlorocephalomannine (I) obtained from this invention in a
screen of sixty human tumor cell lines. Figures 25A-25F
represent mean graphs of dose response of (2"R, 3'S)
dichlorocephalomannine diastereomer (I) obtained from this
invention in a screen of sixty human tumor cell lines. Tables
19A-19F show data sheets of in vitro testing results of the
(2"S, 3"R)-dichlorocephalomannine diastereomer (II) obtained
from this invention in a screen of sixty human tumor cell
lines. Figures 26A-26F represent mean graphs of dose response
of the (2"S, 3"R)-dichlorocephalomannine diastereomer (II)
obtained from this invention in a screen of sixty human tumor
cell lines. Figures 25A-25F and Figures 26A-26F show strong
antitumor efficacy for both of these compounds.
i
CA 02210924 2002-09-06
- 68a -
The invention also relates to a pharmaceutical
formulation which comprises any one or more of the dibromo or
dichloro diastereomers of the invention or a pharmaceutically
acceptable salt thereof associated with one or more
pharmaceutically acceptable carriers excipients or diluents
therefor.
CA 02210924 2002-09-06
- 69 -
0~ ~ O C- CO CU ~ ~ ~ n ~ 00 M 0~ ~ U7 --~ .~ Il~ M
M --wD vD O O I~ CO M ~t CU vD 0~ ~ CU Lf~ (L! (~ CU M O~
.-~ ~ U7 U7 00 ~ U7 C' 00 M fLl M .-. O .-. O O O
I ~ O O O O O O O O O O O O O O O O O O O
C I
O
._ d- <T ~- 0~ M ~-~ (L1 0~ ~ U7 (U vD o CLJ (1J M M 0~ CLl M
+' <n M (U U7 00 W D N M (1J f~ n .-~ vD U7 (L! ~ (LJ ~ ~ lf~ ~
d p~ - Q~ M CwD I!7 d- I~ U7 0 00 v' U7 (U .-~ (U .-. .-. .-. ~
L ._ ~ . . . . . . . . . . . . . . . . . . . .
-~' +, I O O O O O O O O .-. O O O O O O O O O O O
C ._
Q~
U c t\ ~ ~ 0~ 0~ ~ In U7 vD 00 n vD 00 O~ M ~ CLI O vD n
C ~ M tf7 .~ --wD O 0~ 00 1'~. O vD vD (U ~ O~ (LJ v0 ~ ~ (U Lf7
0~~-U7~-~U7~ ~Il~.~O~(W D(U ~M.-~~(UM(U
U ~
I O O O ~ O O O O .-. O O O O O O O O O O O
o_ d
m U ot~.ocULl~N 0~(LJOMII)~o vDU~MU7t~W D
oovtD~cM-I~ ~~(~tl~'oNO°°~
o,~ ...... ....... .......
I .-roo....oo ..-~o.~.-.o.-.o ooo.~ooo
c
pp d _
g M ~D ~t 00 ll~ --~ W D tf7 ~ M 00 ~ ~ CO 00 (LJ (U ~t I~ 0~
d' (L! 0O Q~ w0 (U O~ M OO O M ~ Q~ O (Ll ~ (L1 M ~
I .~ r., O .--. .-, .--. ..-. O .-~ ..~ .--a ._, ._. O .-. .-. .-~ .~ .-~ .~
~"~O~CW 00(U(U ~'~D~D~'~O.-~~
O~~I~O(ZJO .-,ap(UO(U~M t,l~(LJOOI~OOO~
L M ~-~ 00 ~ wD M Q~ M 0~ O (U M O
U ~ ~ o .-r .-. .-. L .-. o .-. ~, .~ .~ .-» .-. .-~ .~ .~ .~ .-. ...,
N
U
.wD 0~ o o M c I~. 00 (L1 ~ o Q~ 0~ (U ~ ~t v0 ~ N CU
O t~ ~twD ~ lf~ N d U7 I'~ tf7 ~ 00 v0 f~ 1~ M vD ~ I~ 00 U7
i. CUNoM(LI(UUMMv0U7(U~t~-~ .-.~.-..-.~.~.~,-
._ p~ . . . . . . . . _ . . . . . _ . . . . .
OOOOOO~oOOOOOO OOOOOOo
C
J
C
J
N L
-r ~ ~0 U
~ l~ CU vD (U o U U'7 00
I~ f- (1J -~ cLi M N vD c o W D
U U ~ ~- 00 J (1J CU (U CU M ~ d CU 0~ ~ O
\ d I o (U I I d vD ~ Z I I I U (L1 ~ tI7 (LI
E LWD vD i- ~ E X I I I 1 I I p I I ~-~ 0~ (L) ~0
J NQ I LI~J~ v7»~~~~~ cJUI- I CU.-~ I
N ~C U J I O d. ~ I Y O O U U U U O O U U I- t- ~ 3
C ~ U 2 Y ~ Q! cn C LJ I I Z Z Z Z ~ U Z I I Z Y c~
d N O O
~ J Z U
CA 02210924 2002-09-06
- 7
O O O O O O O O O O O O O O O O O
O O O
I
I I I I I I I I I I I I I I I I I
I l~ W l~ W W I
W W W (~ W W W W W W W W C~
W W
O O O O O O O O O O O O O O .~
O O ~ ~ O O
O O O O O O O O O O O (1J O ('~ ~
O O ~ O O
U
J U7 tf7 U7 Lf7 U'7 U7 lf~ --~ Lf7 --~
U7 tf7 U7 Lf7 U7 U7 ~t .-~ fl)
lf~
n n n n n n n n n n n n n n
n n
U7 U7 U7 U7 tI7 U7 lf7 L>7 I~ tf7 IW D
U7 U7 lf7 UW D vD vD v0
O O O O O O O O O O O O O O O O O
O O O
I I I I I I I I I I I I I I I I I
I I I
W W LJ LJ W W l~J W W w W ~J VJ VJ
w w W W LJ L~
000000 OOO~p[f~yj'7 .--~(U(L,~OO~.CQ
000000 ooooM(U~ (Ud-o.~.~~.-.
U . . . . . . . . . . . _ . . . . .
. . .
I- ll~ U7 I!7 U7 U7 U7 --~ vD In U7 vD
Ilk l!7 lf~ M -wD lf~ IW D
nnn~nn nnn
U7 00 tf7 vD I~ U7 n n 00 00 00 I~
n - 00 n 00 00 OJ 00
0000 0 0000000 0000000
I I I I I I I I I I I I I I I I I
I I
W w w w W w W W w W W 1J W W W W
W W W
'
0 oppoM .-~ ap~ooo~tn ~(
70~o~.-ro
U7 o N o .-~ o .-~ N N N M 00 ~ tf7
~ tw M M 'D Q7
l~ tf7 (U U7 .-~ CLI --~ M 00 CU (L1
I~ M (L1 --r ~ M (U (U M
n n
M vD d~ --~ M Q~ ~ tf'7 ~- Wit' lf7
00 ~' (L1 ~ I~ f'~ CU IW D ~
- f~ (U vD vD (U (1J ~t I 00 M DO tf7
.-~ M 00 (lJ M
d- I I I I I I I I I
I I
M Q~ M (U (1J ~ U7 M N 00 0~ ~ lf~ I!~
v0 00 (U v0 --~ 11-
ll~ -~ lf'7 M CU vD (LJ fLl (U
CU M ~ ~ ~
lf7 I I I
I
c
O M (LJ 00 U7 In (L1 I~ 0~ M o o .-.1
U7 W 0 (L1 Q~ M 'p .~ o
._ . ~ .~ ~7 ~7 lf~ M v0 (lJ
M I ~ -- M -
+' vD
d I
L S
c 3 M (UOOwO--~N M--f~OD~nI~ -~M.-~I~~tM
N O - I~ .-wD 00 OD 00 00 CO W D CU 0~ (U
(L1 I I~ C~0 M (''~ V
U L f~
c L7 I
O
U +'
c M r~clJ--~~~M tl7l!'7001!'7~~TO(UU7MM00--~0
o ~ - o.-~o.-~~ ~~p~~oo.-. p~p~.-.o~~o
.-. U ~ ..~ ..~ .~ .-. .-. .-~ ...~ ~ .-.,
.-r
I
O Q~
J ~
i ,
CA 02210924 2002-09-06
- 71 -
o p~ M M --~ N Lf~ ~t M ~- N --~
M I~ W D 00 II7 M
00 M O f~ 0~ Lf7 ~ M W U7 Wit- ~
Lf7 ~ D I~ ~ 0~ M
~D ~ N M o M o .~ .-. M dwD ~ N
d- ~ ~ .-. ~
O O O O O O O O O O O O O D O O
O O O
I
vDNt~~Il~o ~U7~~DI~O(U ~NO~OO.-i
oO~NvDo-- wDM~CTMMo OOvO~--~Lf7~o
U7 .- N ~t vD ~t N M N W 0 O~ I~
lf~ N 00 v0 M .~
O O O O O O O O O O O O O O O O O O .-.
O~~OO~MM vD--~MI~00--wD ~N~oU7vD
oI~00N--~M Nl~~tol~N~- ~I~-~O~tf7M
vDl~~tf~00N I~U7~If~NW D U7vDoI~MN
000000 0000000 00.-~00.-~
NI~MNMO~ ~~U7~D(UMO~ Of~NOW DM
0~000~~~~ U7~Nlf7o('W 00~-00~00~-
0
r.NoQ~W D --wDO~~MNo OOoN--~000~
or-..-.ooo .~oooo~.-r o.~~.--.o.-.
''I n00N1~.00~ NvDU7f~o00w D~M~o_.~
vD vD ~t ~ ~ mD vD ~ 00 I~. o M III Q~
.- .-r Q~ 00 0
O~M~t--ro0~ OOOONO~U7~M ~-MMNoo
o.~.-..--..-.o .--~o.~oo~~ r..~.~.~.-.(U
O ~t vD ~ M 00 o N Wit- U7 vD o ~
M 00 tJWD N vD
M W D Cb f~ .~ .-. ~ M ~- 00 ('~ .-
M .~ I~ o v0 III
o d- ~t --~ I~ Q~ M o U7 ~t M M M
O ~ ~t ('~ .-. o
.-.. .-, .-.. .-. o .-. ..-, ..... ...,
.~ ~ o o .-, .-. .-. .-.
.-. N
Q~~M~('~f'~ ~Mf~NNvD~ M~tOI~U700
ll~U7~tMMll~ 1f700~~W Dv0 oO~MU7Nv0
N('r7~MLl7--, ~NNtf7~~M ('~~~~tNvD
000000 0000000 000000
U
00 C
L ~~ NNI~ d
Q~ >M I I U7NU~MV U700M
UGOU7~O~LI7 d~ I J_IN~D > I I I I I
C vD O~ ('~ ~-~ i~~ ~ ~--~ t~J L~J L~J I I C O Q ~ ~ ~ >
d N N tn I I .--i O ~ ~ ~ U U d I Q Q Q Q O
U I I I L~7 ~ U7 C X J V' I I U U -- ~ U U U U I
l~ L~ L~ Z Z N d D Q ~ Y Y Q 4 L l~ > > > > Y
t/~cnc~c/~cnt~Z-~J~~c~vWU d~~~~Oc~
Z N >
U ~ D
CA 02210924 2002-09-06
- 72 -
lf~ U7 vD U7 <n Ltd U7 U7 U~ U~ Lf7
U~ U~ U7 U~ U7 U~ U7 U'7
000000 0000000 000000
I I I I I I I I I
I
I t I I I t I I I
L~J W W l~J W W ~J W W W L~ W W W W W
~J W W
ooppooo oop~.-.ooh 000000
0 o M o 0 0 0 0 .-.~ <t7 o 0 0 0 0
0 o M 0
U7 ~ 00 Lf~ U7 117 U7 M - U7 Lf7 lf7 U7
U7 U7 ~ U7 l!'7
tf7
n n n n n n n n n n n n n
n
LW D I~ UW 0 U7 U7 vD I~ U~ tl~ U~
vD U~ U7 U'7 U7 U7 II7
000000 0000000 000000
I
I I I I I I I I I I I I I I I I I
VJ ~J W W W W W W W W w L~ I
LJ W L~ W LJ
w W
o~~o~Cp ooo~oo~ 000000
o~oootw -oppNooo oMOOOo
U7 .-wD U7 W .- tf~ M ~t U7 N U7 t!'7
D U7 U'7 -~ tf~ tf~
n n n nn n nnnn
I~ 00 t~ I~ 00 t~ t~ 00 I~ 00 IWD t~
00 I~ f~ I~ r~
00000 0000000 000000
q I I 1 I I I 1 I I I I I i
I
wwwww wwwwwww wwwwww
H ~tW DIW D U7o.~QW DNA oM00~lf~.-.
OO~lf7MIW DMM.~~tM~ I~.--..-,0.-~~7
N00-~Il~f~ ~MO~~M~-~ ~'~OOM.-~M
H
O.-.(U~('7o0.-.~oo~~pp~
U7 vD tf7 ~ ~t ~f .-w0 I~ l!~ -~ M .
I I I 1 I I t I I
NU7~U71f~1~. M~tNI'~MO~~ U7oU'7U7fW
D
Md-d~- I MN I NNMN ~NU'7Mr.-.
I I I
U700~NNo tW DOOvDM0000 Wit.-wDO~~t.-.
~M NU~-- M~-m Md-N NNvDM-~~
OW D ~ n M 0~ d~ U7 (U --~ OW 0 00 00
0~ --~ o .-~ N
~D 00 vD n 00 D W D d- M 00 ~t vD 00
LlW I~ 00 t\ ~
(LJMOONU7f~ O~M.-.oU7o.-, MO~U70~wD
~0~Q~0~Q~0~ oO~Q~Q~o00 0~0~oQ~0~0~
.~ .-.., .-
CA 02210924 2002-09-06
- 73 -
('W D I~ -- 0~ O~ CO ~ ~ U~ O
~ O CLI U~ vD I~ ~t
W OLf7--~~(UOf~ ~~ CO(LI--~CU~0~00~
CU vD -~ I~ .-. ~ .~ M CL! (L1 ~
.-~ (r7 l!'7 O O (u vD
O O O O O O O O O O O O O O O
O O O
I
(1J I~ U7 ~ O (~ ~D vD C''7 .-~
O M -~ CO lf7 ~ ~ II7
('~ 00 ~t O ~ O UW D 0~ O (U U~
CO V~ 0~ ('~ 00 U7
C~00U7OO('~~W D-- LfW DV~too~~
O O O .-~ .-~ O O O O O O O O O O O O O
ll~ (L! C' tl'7 Q~ N Lf7 ~t M t\ I~ ~--~ ~' 00 ~ M (1 00
OOvDo00(UMO~~ I~00 vD(~~~t-~tvD(U~-
vDW DQ~~~~tI7 I~(~ LT7o~U7oo0~0
0 0 0 0 ~ 0 0 .-~ 0 0 O .-~ 0 0 0 0 0 .-.
vD~t~(70~00--~fW Df~ C'~OvDvDo('~W D
00 ~t .-~ C'~ tJ~ ll~ t!7 o I~ (L1 00 vD ~ I~. M --~ t~. Lf7
V'.-.oU7f''7~I~D0 ~o (~('W DI~.-~(UOV-
.--, .-. .-. .-~ .-, O O .-m-. .-~ O .--. O O O O .--~ .-,
0~('~~I~II7~I~t~ (W D vD~-'1f7~'~-~Opv(D _
~' lI7 ~D ('~ ~ 0~ ~ M I~ CU Q~ l!7 ~1' ~ M 0~ 0~ I~
~D ~--r O In U'7 O f~ 0~ f~ (L1 d' M 00 00 O C''7 ~ I~
.--mr .--i .-a .-w .-~ O .~ .~ .-~ ...-~ ..-r O O .~ .--~ O .-r
O 'D 0~ ~ ~D d' ~ d' lf~ Q~ (U M .'-, ~D N ~D U7 ~
~f~~D~DO~("~~0~ U7~D ~('~~"'~r.O~D~,--'
'D~-'O~D~'~'-~~00 I~.-~ U7~'O~O~~-'~'OQ~
.---~ .--~ ..-, .--a .~ r, O .~ ...~ .--~ .--~ .-, O O .-~ .--~ .-i .-r
00 O I~ ~ M tLJ M vD (U 00 00 ~ f~ ~ (''7 I~ 0~ ('~
I~~o~N00~wD 000 ~CU~U7.-~o~tl~
11~ (LJ --r 00 M M In ~ CLJ CU ~t f'~ M (1J (L1 CU ~
00000000 00 00000000
U
U
L
N ~ Q
U L tlJ \
i. C ~ Q' -~ U7
d U I C7 ('~
U U C Q CU ~t
C M d C~ I t- I
CS ~ ~ ~ III U Q G~ 00 GA O~
U O I M U o ~ +' ~ \ ~ Ice. ~ Z ~t' ~1
I 00 Z ~--~ I fLJ ~--~ (''7 d ('7 ~ +' t\ I~ I In I I U7 I~
-wD 0~ I Y l.~ --~ I I +' I I U1 L~ L~ GC Q Q I ~-
d 00 ~ U 4 X Z ~G O Ut U Z d U U ~ t~ L~ ~ h- I
C I~ Q Q U Q' (W-- ~ O ~ t~ QJ ~ ~ ~ I ~ ~ ~7 I-
N L L
i.
CA 02210924 2002-09-06
- 74 -
U7 LfWn U7 U7 U~ U7 U~ U~ Ll~ !P M (~ U7 1,17
U7 U7 U7
00000000 00 00000000
I I ~ I I I I I I I I I t I I ~. I I
W W W ~J W W W ~J W W W W W LJ W W l~J W
O O O O M .-. O O O O O O O O M O O
O
o O O O ~ ~ O O O O O O O O .-. ~ O O
lf~ U~ lf~ Lf7 (LJ U7 lf~ U7 lf7 lf7 ~ CU Ilk U7
(L1 U7 lf7 U'7
n n n n n n n n n n n n n .
III U7 U7 U7 vD Lf7 00 U7 ~D U7 0~ 00 U7 U7
~D U7 U7 I~
O O O O O O O O O O O O O O O O O O
I I
I I I I I I I I I I I
I I I I I W W W L~J W ~J W W w W
W LJ ~J W W W W
W
O O <J1 O ~ ('~ O .--.O ~ ~ O .-. ~ ~ O
~ O
O O O O [f~ (U O O ~ O .-. 'p O [J~ O Q~ O
O
tf~ (n (L1 U7 0~ U7 Lf7 --~ CO ltd (U U7 M In
~ (U U7 t\
n n n n n n n n
I~ ~D t'~ - i' I~ I'~ ~ ~D I~. (~ ~ C~ ~D I'
~ ~D (~
O O O O O O O O O O O O O O O O O
I I t I
I I I I I I I I I I I I I
W LJ W W ~J LJ w W W W W W W W W W W
(LJ M ~ M O (U M .-wD 00 O O o ~ 00 f~ M -
y D~--mOCLIOdW D~ d-(U~U7o~Ml~
CU n ~ M .-~ CU .-. M .~ .-. .-. .-., ~. ~ .~
~ ..~
H
W
I~ M V' ~ (L1 vD .-. .~ pp CO '. ~ 0~ ~ ~
CU O O
CLJ (L1 N W D ~ !n .-~ ~Y M .~ U7 U7 I
I I I I 1 I 1 I I
I~ I~- ~ --~ N ~ o t!7 U7 I~ CL! O ~t (U vD vD
O ~
--~Lf7Mll7M I CU ~~ CU(U~~I~OOM(lJ
CU I I I
ll7ODvDO~U7I~o.~ M.-.. ~~t(U.-..-~opp~t
M vD ~ ~t ~ M f~ (U (U vD M M 00 (~ f~ M
--~ I I
QW D~~~I~.-~M C0~ -rMU70~~o00Q~
00 0~ Q~ ~ I~ I~ I~ ~- Q~ W D M W D
00 ~ 00 I
.-~(~.oMMU'700vD ~IW DMt~Il~(lJ~t00vD
o~op~op~pp~ 0o ~p~opp~~pp~
i i i ~ i
CA 02210924 2002-09-06
- 75 -
Ml~rwtoU) ~v~oO~O~tf7 U7ocUU7vD00tw
M ~~(UNr.M vO.~W DMoM oNor.vO~t\
M--~.-~NN~ U7MI~.U7Nv~ o.-~ooo~lo
. . . . . . . . . . . . . . . . . . _ ,
I 000000 0000000 0000000
C I
O
OWOO~~Lf7~ W D~NI~M~ Mo.-,~O~U7(1J
M M00NIW DM ~N~'I~~tJ~~ ~~00--~CU0000
d - Mr.~~-»d-MN 001I~vOMU7N ~-~NOCVNM.-.
L ~ . . . . . . . . . . . . . . . . . . . .
'f'' I O O O O O O O O .-. .--. O O O O O O O O O O
C
0~
U ~~MNoN NvOOW D1~MM v0U7~NOW 0vD
C M ~I~I~U70"~ .-..-.~t,~ll)~U7 vDO.--w00~M~
O - M .-~ ~. v0 ~ ~' o lf~ o .--~ W 0 (1J --~ d' .-~ lf7 (L! ~ ~-
U ~p . . . . _ . . . . . . _ . . . . . . . .
O I O O O O O O .-. O .-. .-r O O O O O O O O O O
rwocv0~a~ ~U7o0~.-~NU7 .~I~O~Nootn
O M ovt(LIf~NM oov~pr,r-.M MO~O~~NoM
wON~~~lf7 NI~.NU7v0o~t MU7.-~O~W Dot
. . . . . . . . . . . . . . . . . . . .
I 000000 .~0.-~.~O.~O 0000000
('~Lf~0o00M W Of~~00~p 1f7U7oM1I7in~
"! M ~~--~U7n.~ o(U~~~-~vOlw t~...,.-.U7N.-...M
oO~IW DO~~ NO~NQ~o(LJN O~oooNM.~
~ O O ~ O .-r .-w O ~~r ~ ,~ .-r .r O ~..w, .--~ .~ .-, .~
~o~tO~p~ ..~~ODvDoNN W 0.~.-,O.-,~
OO~tf~.oNo .....ppNoNO~M If7Noof~000~
L M~-~CO~--wD M~MO~oNM o.-~~,...~J~.~
. . . . . . . . . . . _ . . . . . . . .
U ..~ .-. o .-~ .~ ~ L ~ o ...~ ..-. .., .-.. .... .-. .... .-. .-, .-. .-, .-
,
U
~OO~OOM CI~~N~~OO~pv N~'vtvD--~N(LJ
U1 O !~ ~t v0 0~ In N d U7 t~. lf~ ~ 00 v0 1~ I~ f'W D ~ Iw 00 tl7
i NNoMN(UUMMvDtnN~--~ -~»CLI.-~.-..-..-..-.
._ ~ . . . . . . . . . . . . . . . . . . . .
+.~fV 000000 X0000000 0000000
'- C
C 0~ J
4J C
A ._ _.
J -'
"' ~ i
U~ ~~1 N U vD NO Utf7~
- ~ l.a.I- N ~ NMN~ CoQ~~D
+'U U~ X00 -- NNNNM~ dNO~.-' o
Cl\ d I 0N I I d vDO~====U N--~Lf~ N
O E LW O v0 1- ~ E X I 1 I I I I O I I ~-~ 0~ (LW p
N Q~ ! !l7 J f fn » ~ ~--~ ~-~ ~ .-. c J U H I fU ~-~ I
~~CUJ I O~~ I Yf~L~UUUU O~UU!-E--~3
D C ~UZY~~t/7 CI~JZZZZZZ-'UZZIZYCn
E~J Z U
i
CA 02210924 2002-09-06
- 76 -
U7 lf7 U7 U7 U7 U7 U7 lf~ Lf7 v0
U7 U7 U7 U7 U7 (f7 U7 tf7 U7
tl~
000000 0000000 0000000
I I 1 I I I I I I I I t t I I I I
1 1 t
wwwwww wwwwwww wwwwwWw
0 0-0000 0000000 If7o...o~oo
000000 0000000 Notnoc~oo
U . . _ . . . . . . . . . _ . . . .
. . .
J U7 ~f' t!7 U7 U7 U7 U7 - U7 ~f' U7
Lf7 U7 U7 U7 U7 U7 ('7 U7 ltd
n nnnn nnnnnnn n nn
ll7 ~ tl7 U7 tl7 lf7 tf7 fW O W D v0
vD lf7 vD lf7 U7 ~D lf7 -
o O O O O O 0 O O O O O O O 0 O O
O 0
I 1 I I I I ! I 1 1 I I I I I I 1
l I wwWWWW
WWWWWW WwWWWWw
o~poNo~ oc~oNr~00N C~7of~v~r~o
.-. o~po~po(~7 orTo~~(~7~p ~~p~-pptTo
. . . . . . . . . . . . . . . . .
. .
H- U7 ~ U7 v0 lf~ -~ U7 ('~ ('W D vD v0
!n tl~ --~ -w0 tn lf7
n n n n n n
OD~~t~~W DW Df~WI~~ OOt~~f~r~~00
000000 0000000 0000000
I I I I I I I I I I I I I I I 1 I
I I I
~ ~ ~ ~ m
O ~ U7 r~ U7 CU ~-~_.QW O v0
U'7 '~ ~ (L1 fW D
U7 U7NW DvO~ -~~wt'~(U-~o I~~~'ll7--C'~N
. . . . . . . . . . . . . . . . .
. . .
l' ~ .-~ ('~ ct vD t\ M v0 -~ C~ --~ M
N .-~ .-. -~ N N N N
('7 ~(''7t~.("7NQ~Nl~~tf7U7(''7~toppoO~N(''7~
- 1I7 ~ c7 cU -~ .-~ I o ~- o wD
~ ...,. N
d' I I I I I I I --~ I -~ I
I I I
1 I
M vD ~ IW 0 ~ ~ ~ f~ d~ I~ I~ .- OD
('~ .~ o t"7 U7 v0 ('~7
N ..~ ~t .-.. lf7 .-.
('~ -, ...
tl7 I I I
I
C
O t~7 --~ O~ f'7 0~ ('~ U7 Lf7 ('~ 0~ o a-
~ I~ ~ d- .- vD N O vt
._ . .~N....~,.~...~~pN~p~(ZJN I -('~~---.N
I
+' v0 1 I
d I
~ S
c 3c'~ oCr7~t~oN O~c'~-~cW DcLJ 00--'~~c'~(Uf~
N O - t'~ I d'('~NNtDlf7~f~U7v0N -~t' ~N("~N
U L f~
c t~ I
O
U +'
c("7 0~('~oO~W ODoll7C"70W vD~oU7v01~~
D Olf7
wDOD0000C000 O~O~Q~o~O~~ CJD000~0~0~~0~
U~
~ ~ I
O <L
o~
CA 02210924 2002-09-06
_ ') 'j _
ot~oNO~o O~~U7(UO~fJOQ~ .-~o~.~fW
D
(~. .-. ~t 00 M .~ 0~ ~ W ll~ 1~ W
Q~ t' D o D o U7
M.-.,.-,r7U7o oMo.-,..,~.~ ~MU7MNo
000000 0000000 00000.-.
f~.O~~nMM oU7~U7~U7tn Mot~tl7N0~
vDvDt'~U7No vDf~OW OvD~o ODMO~OOODN
If~NN~I~N lntnN~NODvO vDU700f~M.~
000000 0000000 00000.-
o~o~.~o IwMoMNOU7 N~~MOO~t
U7 vD DD W D o t~ I~ o o M --wD vD
t~ U7 ~ U7 --
vDvDW GOON f~.v0~U7MOW I~U7~O~MN
0
000000 0000000 ooooor-.
U7.-~O~NO~W DOONU7NMN f~~t~00U7o
-~I~O~W DU7 W D--~V'~M~ I~NvDIJ~MvD
~Mt~l'~0~~ O~I~r~~M--~ ~~o-wOU7
o.-.oooo ooooo.-.o oo.~.-.o.-.
H
~~OOM~tN vDtf~NU7l'~M~ ~twD--~MU7
O~ U7 et .-. yD I~ o U7 M v N t\ ~
.-. 0~ N (r1. U7
Q~U7M.-~OW O~MoU7~tM MNNN--'o
o .-. .-~ r, .-. o ... ...,
.-, o o .-. .-.
O~tvD~MM OOON~ODU7vD U7vDO~N~
MWOOOI~M .~~~M~t.-~t~ ~M--ovDU7
0~~~00~ nO~Mo!!~~-M ~MMM.--.o
.-. .-. .-. ~. o .-. .-~ .-. .-. .-,
..-. .-. o o .-. .-, ..
~--~M~Mf~ ~MI~NN~--r M~'Of~lnm
tnU7~MMtf~ tf~~~~~~~ o0~MU7Nv0
NM~'Mln~- ~NNU7--~YM MV-~~NvO
000000 0000000 000000
i
U
c
i ~~ NNf~ d
>M I I lf7NU-~M~tU7~M
U~U7~Q~U'7 d~ I JJN~D > I I I I I
cvD~M.-~t~ E~La.J L~JI~J I I cp~~~~>
dNNU7 I I .~ O ~ ~~UU d I aaaap
U I I I G~lf~ilf7 cXJ~ t I UU --Q'UUUU I
L~l~.t~.ZZN dpQ--~YYQ<C ilk»»Y
cnc/lc~c~c/7c~~-J~~c~c~~~ d~ppOpc~
Z N >
U ~ p
i
CA 02210924 2002-09-06
O O O O O O O O O O O O O O O O O
O O
I
I I I I I I I I I I I I
W W W W W ~ I I I I I I
~ W W W W W W W W W W
~ W
OIf~Q~00d' hOhQ100h 000000
oo.~ooo ppo(~~oo0 000000
tI7 N ~ U7 tI7 N In f'7 .- tl7 lf7 U7
~?' U7 tl7 t;'7 tf~ U7 U7
n nn n nn nnnnnn
tl7 ~ h lf7 vD N vD h N tf7 vD tl7
Il7 v0 lfW 0 l!7 t!7 tl~
000000 0000000 000000
I I I I I I I I I I I 1 I I I I I
w w tsl W lsJ I 1
I,.vJ --~ lyJ L~J l.J LrJ LJ
l.~J L~J t~J t~J L~! lyJ
LJ
opvppoo(~ r-.op~('~~po.-Ø-.0(100
ohoooN oom~moo oooNoo
If7 -wD U7 U7 0~ Il7 U7 ('7 tf7 h U7 N
vD --~ U7 0~ U7 tf7
n nn n n n n nn
hh~W D~ W D~~hh~J ~W Ohh(~
000000 0000000 000000
1 I t I I I I I I I I I t I I I I
I I
t~J W w t~J W l~J W W t.~.JL~J W l~l
ts.l W l~J W w w t~J
wO.~M.~~ O~oMW 0(''7~ oU70v0I~.h
" '
~f'W DvD(~~ U7ovD( 00~.~t
NW ~Q000
tI~ N N ('~ ~-~ ('~ ~t M U7 h ~-~ v0 tl~ ~ N v0 ~- ~-. ....
~hOW ONvO ~U7.-~~rc~oo Ntf~00~o000
~--wD v0 ~-~ U7 h vD h N h --~ N ~ N I N
I I 1 1 I I I I I I
o~0~~ttf7v0 vOhU7~t.~o~ N~f'~O~ht"7
~?'N('~~~t'~ N~ -~('~Q'N ("7 U7('~~~t'~
I I I
.-.p~~tU--~U7 tl7Noh0~-~c'W Dc~oOD~tcT
U7 N t'7 vD ~ ('W 0 N W'7 U7 f'~ (''7 vD ~ ~--~ ('7
N('~U7('7.-~~ ODhNNO~o~-~ Q~O~f"7("7~tC
h0~f'7~~f''~ ~?'hllW Dlf7hlf7 ~('~hODW D
U7.-~O~NQ~lf7 ht"7('~~NNtn ODvDvD~ho
O~~~O~(D~ O~~o~oo~ OO~O~Q~O~c
i
CA 02210924 2002-09-06
_ 79 _
N0~--~COvDNNC''7MIf W DN('"7('~tl7~N~
v~ U7 ~ ('7 0 wD f~ ('~ 00 0 ~t
('~ ('~ ~t ~ .--, .-, 0~
N vD --wD -~ ~ ('~ M .~ tf7
.-. ('~ Il7 .--~ 0 .- N t~
00000000 00 00000000
I
o(n~W D~tf~00 I~o OONoNoc~r~N
O~OI~d'OONNN~- .-~N t~U7U700t'~Ii~('~~
(~ pp U7 0 0 ~p lf7 Iw ('7 U~
~ ~- O~ .- o o U7 0
ooo.--,.~oo0 00 0000000.-.
vD0~o1~0~~(''700 00~ U7t~N0~~o~~t
vDovDU7W Do~ C~I~ OO~v~CW 0.--~.~
~o~poo~U7~p r.N ~o~~poot\.-.
o.-..o.-..~oo.-. 00 0.-00000.-. '
.-.ooyDMp~.-~ 1~--~ c~7lf7NNO~I~00o
oc''~.~oU7.-~No ~.-. OON~ovDlf7Q~U7
('~ ~.~ o lf7 N vD vD O~ o PwD d- 117 00 0 .-~ 00 ('~
.--i ~ .--~ .~ .-r O O .-~ .--~ O O .~ O O O O O rr
MU7NODOOI~oI~ U7o 117.-~U7l~.~o('~U~
t~t'~~U7('7--~I17.--~ 1I7~ Nf~.-~o("71f7('~~ -
~.-.o~p~~~00W 0.-' ~('~~O~I~.~Of~
.-~ .--i .-.~ ..-r .-r .-r O .-~ .-~r .-r .~ .-r O O O .-~ .-r .-r
o v0 00 vD v0 ~ 00 ~t U7 0~ N M .-w0 N vD U7 O~
~J" IyD vD O~ ('~ ~ O~ llW O ~ ('~ ~-~ f~ O ~O O~ ~--
lD ~~ o ~p ~ ~~ 00 W I~ .~ U7 ~t' 00 0~ ."'~ ~ O OD
.~ .--r .~~ .-n .--v .--~ O .~ .-r .~~ .~ .-, O O .-r .-~ .-r .-~
00or.p~('~N("7v0 N00 0~~t~0~t'7f~Q~('~
I~--~o~N~yO CD00 ~N~tlf7.-~oQ~U7
--~II~N~~('7MU7 ~N N~('~('~NNNvD
00000000 00 00000000
U
U
L F
cn a
a ~ 1.~ \
L c ~ Q' ~-~ In
d U I ('7 ('~
U U c ~N
c (''7 d A I !- I
d ~-~ 0~ N In U 4 fzt 00 ~ 0~
U o I ('7 U o ~ +' ~t \ ~ I~~ f Z ~' f~
I ~ Z ~ I N ~-~ f'7 d ('7 ~ +' (~ 1~ I Il7 I I Lf7 f~
-wD ~ 2 Y LL .-. I I +~ I I ~n 1.~ 1~ a a a I ~
d~d'UQXZYO U1U~ dUU~lf/)AAi- I
c f~ Q Q U ~ fn I- U O Q. ~1 ~ ~ f f = ~ ~ G4 H
L L
CA 02210924 2002-09-06
Lf7 lf7 lf7 lf7 lf7 lf7 III ll~ '
lf~ lf~ U'7 ' lf7 00 lf7
U7 t!'7
O O O O O O O O O O O O O O
O O
I I I I I I I I I I I 1 I I
I W W W W W W I
W W W W W W W l
W
O O O O .-r (~ O O O O O N O
O O O
O O O O ~' O O O O O O ~Q O
O O O
U7 U7 lf7 U7 U7 U7 U7 ll7 U7
N ('~ U7 U7 U7 ('7 U7
n n n n n n n n n n n n
-
tf~ U7 U7 U7 v0 U7 U7 tl7 vD tf7
v0 U7 lJ~ n ~ ~ U7 U7
00000000 00 00000000
I I I I I I I I I I I I I I I I I
wWwwwwwW Ww 1
WwWWwwww
oO~o(~7pptwp I~~p om(~o~~oo
oor.oop~(~ly ppp o~....o~pp~po
U7 Ilk --~ U7 O~ N ('7 lf7 --~ lf~ U7
tn ('7 f'~ ~ N ~ U7
nn n n n n
fW D fW 0 f~ 00 OD 00 vD 00 I~ Q~
00 vD 00 0~ 1~. t~
00000000 00 00000000
I I I I I I I I I I I I I I I I I
C~, W W W W W W lJ VJ W VJ I
W W W W W W W
W
a1 I~OD~~tf7~Q~o .-~p~ O~~C.-CnNN.-~N
0 vD 0~ .-~ o lI7 00 U7 In N f~ 00
N 0~ tf~ 0~ ~ U7
f.~ N I'~ I~. 00 N N ('7 N -~ In ('7 tl7
O~ -~ ~t- OW D
a
~a
~NN~t~U7('~~ ~O~ o0~l~c'~~f~O~N
Nc'7NODvD I I I C' .-~Nb-NI~~fN~
I I I I I 1 I I
W 0 o I~ o vD v0 .~ D t''7 .-wD vD
O~ py ~ o (''7
--~U7vtU7r7 ~N ~U7 N(''7 ('~00n('7M
I I I
t"7tf7t~U7.~--~N~ ovD vOt~OrN.-No
('~f~U')U'7~--~("7f~N I NvDN~f~f~ln~'
I I
f~ t'~ I~ ~ ~ -- ~ 00 ~t 0~ .-~ N OO
f~. .-. dW f~ 0
n0~0~00vD('7U7o ~~ ('~~tf~fwONtW
O
I I
tI7~NQ~--~~o~ N~ --~~--~O~ODU7N1~
O~O~oO~O~Q~O~ ~0 0~0~o00tf7n0~0~