Note: Descriptions are shown in the official language in which they were submitted.
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
PROGNOSTIC BIOMARKERS IN PATIENTS WITH OVARIAN CANCER
RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application Ser. No.
61/406,044, filed October 22, 2010 the entire contents of which are hereby
incorporated herein by reference.
BACKGROUND OF THE INVENTION
Ovarian cancer is among the most lethal gynecologic malignancies in
developed countries. Annually in the United States alone, approximately 23,000
women are diagnosed with the disease and almost 14,000 women die from it.
(Jamal,
A., et al., CA Cancer J. Clin, 2002; 52:23-47). Despite progress in cancer
therapy,
ovarian cancer mortality has remained virtually unchanged over the past two
decades.
Given the steep survival gradient relative to the stage at which the disease
is
diagnosed, early detection remains the most important factor in improving long-
term
survival of ovarian cancer patients.
The poor prognosis of ovarian cancer diagnosed at late stages, the cost and
risk associated with confirmatory diagnostic procedures, and its relatively
low
prevalence in the general population together pose extremely stringent
requirements
on the sensitivity and specificity of a test for it to be used for screening
for ovarian
cancer in the general population.
The identification of tumor markers suitable for the early detection and
diagnosis of cancer holds great promise to improve the clinical outcome of
patients.
It is especially important for patients presenting with vague or no symptoms
or with
tumors that are relatively inaccessible to physical examination. Despite
considerable
effort directed at early detection, women generally present with disseminated
disease
at diagnosis.
Thus, there is a critical need to identify one or more panels of biomarkers
that
deliver the required sensitivity and specificity for early detection of
ovarian cancer.
Without an acceptable screening test, early detection remains the most
critical factor
in improving long-term survival of patients with ovarian cancer.
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
Although the stage of disease is one of the strongest predictors of survival
in
patients with ovarian cancer, disease stage alone is not adequate to predict
survival or
outcome in these patients. Improved methods for predicting a patient's
prognosis
could improve patient management by, for example, identifying patients in whom
more aggressive therapy might be warranted or to whom personalized treatments
might be offered..
Thus, it is desirable to have reliable and accurate methods for determining
the
ovarian cancer status of a subject, predicting overall survival of a subject
or predicting
progression free survival of a subject. The results of such methods are useful
in
managing subject treatment.
SUMMARY
The present invention provides compositions and methods for determining
ovarian cancer prognosis (e.g., predicting overall survival probability or
predicting
progression free survival probability). Such methods are useful in selecting
an
appropriate therapeutic regimen for the subject.
Advantageously, the invention provides compositions comprising one or more
biomarkers and sensitive and rapid methods for using the kits to determine the
survival status of patients with ovarian cancer by measuring the levels of
particular
biomarkers in a biological sample. The detection and measurement of these
biomarkers in patient samples provides information that diagnosticians can
correlate
with survival status of human ovarian cancer patients or a negative diagnosis
(e.g.,
normal or disease-free). In one embodiment, the markers are characterized by
mass/charge ratio, molecular weight and/or by their known protein identities.
The
markers can be resolved from other proteins in a sample by using a variety of
fractionation techniques, e.g., chromatographic separation coupled with mass
spectrometry, protein capture using immobilized antibodies, bead-protein
complexes
or by traditional immunoassays. In preferred embodiments, the method of
resolution
involves Surface-Enhanced Laser Desorption/Ionization ("SELDI") mass
spectrometry or immunoassay.
In one aspect, the invention generally features a method of determining the
prognosis of a subject having or suspected of having ovarian cancer, the
method
involvingcomparing the level of biomarkers inter-alpha (globulin) inhibitor H4
(plasma Kallikrein-sensitive glycoprotein), transferrin (TFR), and beta-2
microglobin
- 2 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
(B2M) or fragments thereof in a sample from the subject to the level present
in a
reference, wherein an increased level of said biomarkers relative to the
reference is
indicative of a poor prognosis.
In another aspect, the invention generally features a method of determining
the
prognosis of a subject having or suspected of having ovarian cancer, the
method
involvingcomparing the level of biomarkers B2M, TrF and ITIH4 or fragments
thereof, wherein an increased level of said biomarkers relative to the
reference is
indicative of a poor prognosis.
In another aspect, the invention generally features a method of determining
the
prognosis of a subject having or suspected of having ovarian cancer, the
method
involvingcomparing the level of biomarkers B2M and CTAP3 or fragments thereof,
wherein an increased level of said biomarkers relative to the reference is
indicative of
a poor prognosis.
In one aspect, the invention generally features a method of determining the
prognosis of a subject having or suspected of having ovarian cancer, the
method
involvingcomparing the level of biomarkers CA125, HEPC, B2M and CTAP3 or
fragments thereof in a sample from the subject to the level present in a
reference,
wherein an increased level of said biomarkers relative to the reference is
indicative of
a poor prognosis.
In one aspect, the invention generally features a method of determining the
prognosis of a subject having or suspected of having ovarian cancer, the
method
involvingcomparing the level of biomarkers AP0A1, TT, HEPC, B2M, CTAP3, TrF
and CA125 or fragments thereof in a sample from the subject to the level
present in a
reference, wherein an increased level of said biomarkers relative to the
reference is
indicative of a poor prognosis.
In one aspect, the invention generally features a method of qualifying ovarian
cancer status in a human involving providing a subject sample of blood or a
blood
derivative; and fractionating proteins in the sample on an anion exchange
resin and
collecting fractions that contain inter-alpha (globulin) inhibitor H4 (plasma
Kallikrein- sensitive glycoprotein) (ITIH4), transferrin (TFR), and beta-2
microglobin
(B2M).
In one aspect, the invention generally features a kit containing a capture
reagent that binds a panel of biomarkers containing, inter-alpha (globulin)
inhibitor
- 3 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
H4 (plasma Kallikrein-sensitive glycoprotein) (ITIH4), transferrin (TFR), and
) beta-2
microglobin (B2M); and a container containingat the panel of biomarkers.
In one aspect, the invention generally features a kit containing capture
reagents that binds the panel of biomarkers fragments containing inter-alpha
(globulin) inhibitor H4 (plasma Kallikrein- sensitive glycoprotein) (ITIH4),
transferrin
(TFR), and ) beta-2 microglobin (B2M); and instructions for using the capture
reagents to detect the biomarkers.
In one aspect, the invention generally features a system containing a
plurality
of capture reagents each of which has bound to it a different biomarker
containinginter-alpha (globulin) inhibitor H4 (plasma Kallikrein- sensitive
glycoprotein) (ITIH4), transferrin (TFR), and) beta-2 microglobin (B2M).
In one aspect, the invention generally features a method of determining an
ovarian cancer patient's prognosis containingdetermining the concentration or
expression levels or peak intensity values of inter-alpha (globulin) inhibitor
H4
(plasma Kallikrein- sensitive glycoprotein) (ITIH4), transferrin (TFR), and
beta-2
microglobin (B2M); and correlating the measurements with ovarian cancer
patient
survival status.
In one aspect, the invention generally features a method of determining an
ovarian cancer patient's prognosis involving determining the concentration or
expression levels or peak intensity values of a combination of two or more
biomarkers
in a sample from the subject, wherein the one or more biomarkers are selected
from
the group consisting of: inter-alpha (globulin) inhibitor H4 (plasma
Kallikrein-
sensitive glycoprotein) (ITIH4), transferrin (TFR), and beta-2 microglobin
(B2M)and
correlating the measurements with ovarian cancer patient survival status.
In one aspect, the invention generally features a method of determining an
ovarian cancer patient's prognosis involving determining the concentration or
expression levels or peak intensity values of inter-alpha (globulin) inhibitor
H4
(plasma Kallikrein-sensitive glycoprotein) (ITIH4), transferrin (TFR), and
beta-2
microglobin (B2M); and correlating the measurements with ovarian cancer
patient
survival status.
In various embodiments of any of the above aspects or any other aspect of the
invention delineated herein, the methods further involve comparing the level
of one or
more additional biomarkers to the level present in a reference, wherein the
additional
biomarkers are selected from the group consisting of apolipoprotein Al,
transthyretin,
- 4 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
inter-alpha trypsin inhibitor IV, transferrin, hepcidin, connective-tissue
activating
protein 3, and Serum Amyloid Al and beta-2 microglobin. In other embodiments
the
methods further involve comparing the level of CA125 in the subject sample to
the
level present in a reference. In another embodiment the method further
comprises
considering one or more of the following: radicality of primary surgery, age
at
diagnosis and treatment. In other embodiments the method further comprises
considering one or more of FIGO stage, histological type of tumor, and CA125.
In
yet other embodiments the prognosis is predictive of overall survival or
progression-
free survival. In further embodiments failure to detect an increased level in
one or
more of said biomarkers is indicative of a good prognosis. In yet other
embodiments
a patient's prognosis is used in selecting a therapeutic regiment. In further
embodiments, a poor prognosis indicates that the subject requires an
aggressive
therapeutic regimen and a good prognosis indicates that the subject requires a
less
aggressive therapeutic regimen. In yet other embodiments an aggressive
therapeutic
regimen includes neoadjuvant chemotherapy.
In various embodiments of any of the above aspects or any other aspect of the
invention delineated herein, the overall survival or progression free survival
is
selected from the group consisting of one to two years survival post
diagnosis; two to
five years post diagnosis; and beyond five years post diagnosis. In other
embodiments the panel of biomarkers is measured by immunoassay, mass
spectrometry, or radioassay. In additional embodiments the panel of biomarkers
is
captured using immobilized antibodies. In yet other embodiments the panel of
biomarkers is detected using immobilized antibodies. In certain embodiments
the
correlating is performed by a software classification algorithm. In yet other
embodiments the sample is selected from ovarian tissue, lymph nodes, tissue
biopsy
(e.g., diaophram, intestine, lavage, omentum) ovarian cyst fluid, ascites,
pleural
effusion, urine, blood, serum, and plasma.
In various embodiments of any of the above aspects or any other aspect of the
invention delineated herein, the capture reagent is an antibody. In other
embodiments
contain an MS probe to which the capture reagents are attached or is
attachable. In
other embodiments the capture reagents are immobilized metal chelates. In yet
other
embodiments the kits contain written instructions for use of the kit for
detection of
ovarian cancer status in a subject.
- 5 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
In various embodiments of any of the above aspects or any other aspect of the
invention delineated herein, an article of manufacture containing a panel of
capture
reagents that bind the panel of biomarkers or fragments of the respective
biomarkers
thereof. In yet other embodiments the biomarkers are inter-alpha (globulin)
inhibitor
H4 (plasma Kallikrein-sensitive glycoprotein) (ITIH4), transferrin (TFR), and
beta-2
microglobin (B2M). In other embodiments the biomarkers are inter-alpha
(globulin)
inhibitor H4 (plasma Kallikrein- sensitive glycoprotein) (ITIH4), transferrin
(TFR),
and ) beta-2 microglobin (B2M).
More specifically, it has been discovered that measuring particular
combinations of biomarkers provides a surprisingly accurate prognosis for
subjects
having ovarian cancer. The panel of biomarkers consists of inter-alpha
(globulin)
inhibitor H4 (plasma Kallikrein-sensitive glycoprotein) (ITIH4), transferrin
(TRF),
and beta-2 microglobin (B2M). This panel of three biomarkers has been shown by
the instant inventors to be highly indicative of the prognosis of subjects
having
ovarian cancer.
Moreover, the panel of biomarkers is predictive of survival independent of the
stage of cancer.
The present invention provides a method of assessing an ovarian cancer
patient's survival status in a subject containing(a) measuring the panel of
three
biomarkers in a sample from the subject, and correlating the measurement with
ovarian cancer patient survival status. In certain methods, the measuring step
comprises detecting the m/z (mass-to-charge ratio) values of markers in the
sample
using SELDI.
The instant invention provides methods for determining both progression free
survival and overall survival in subjects diagnosed with ovarian cancer.
Preferred methods of the invention also include assessing ovarian cancer
patient survival status comprising:
determining the concentration or expression levels of the panel of three
biomarkers in a sample from the subject, wherein the three biomarkers are from
the
inter-alpha (globulin) inhibitor H4 (plasma Kallikrein- sensitive
glycoprotein) (ITIH4),
transferrin (TFR), and beta-2 microglobin (B2M), and correlating the
corresponding
concentration or expression levels with ovarian cancer patient survival
status.
- 6 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
In certain embodiments, the methods further comprise managing subject
treatment based on the status determined by the methods disclosed herein. For
example, if the result of the methods of the present invention is inconclusive
or there
is reason that confirmation of status is necessary, the physician may order
more tests.
Alternatively, if the result of the methods of the present invention indicate
a
potentially poor prognosis, alternative or more aggressive therapies may be
warranted.
Furthermore, if the results show a potentially good prognosis, no or less
aggressive
therapies may be warranted.
Examples of more aggressive therapy include: a) The physician may after
surgery treat the patient with more intensive and prolonged chemotherapy. b)
Offer
additional chemotherapy or biological treatments. c) The patient may be
monitored
more closely for relapse or progressive disease. d) Patients with both an
indication of
a poor prognosis and extensive disease, which on imaging indicate nonradical
surgery, may be offered neoadjuvant chemotherapy and subsequent interval
surgery.
e) The proteomic index may be part in the total clinical judgment of treatment
versus
palliative treatment in severe ill patients. f) Radical and correct staged
patients with
stage one and grade 1-2 may be offered adjuvant treatment. g) The patients
must be
selected for surgery by a gynecologic-oncologic surgeon experienced in
performing
extensive procedures Examples of less aggressive therapy include. a) The index
may
be part in the decision making for radical surgery. b) Radical and correct
staged
patients with stage one and grade 1-2 may avoid a potentially harmful
chemotherapy.
c) The patient may be operated by a less specialized gynecologist.
A prognostic index may in the future be used to select patients for
individualized new treatments (e.g. antibody or molecular based). This may be
specially the case were some of the proteins or precursors are targets for the
therapy
The term "ovarian cancer patient survival status" refers to the status of
survival of the patient. Examples of types of ovarian cancer survival statuses
include,
but are not limited to, disease free or overall survival one year after
diagnosis, 2 years
after diagnosis, 3 years after diagnosis, 4 years after diagnosis, and 5 or
more years
after diagnosis. Another type of status is "treatment responsiveness" i.e.
whether a
patient has a high or low likelihood of responding to a given type of therapy.
A third
type of status is "remission" i.e. whether a patient is deemed to be free of
disease (in
remission) or to have cancer after one more therapeutic interventions (in
recurrence).
Other statuses and degrees of each status are known in the art.
- 7 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
For the mass values of the markers disclosed herein, the mass accuracy of the
spectral instrument is considered to be about within +/- 0.15 percent of the
disclosed
molecular weight value. Additionally, to such recognized accuracy variations
of the
instrument, the spectral mass determination can vary within resolution limits
of from
about 400 to 1000 m/dm, where m is mass and dm is the mass spectral peak width
at
0.5 peak height. Those mass accuracy and resolution variances associated with
the
mass spectral instrument and operation thereof are reflected in the use of the
term
"about" in the disclosure of the mass of each of seven biomarkers. It is also
intended
that such mass accuracy and resolution variances and thus meaning of the term
"about" with respect to the mass of each of the markers disclosed herein is
inclusive
of variants of the markers as may exist due to genotype and/or ethnicity of
the subject
and the particular cancer or origin or stage thereof.
A Cox proportional hazards model is a regression model for studying the
association between time to event data and explanatory variables, e.g. tumor
stage,
age and gender. The hazard rate (intensity of the event)on the log scale is
the
dependent variable which is a linear function of the explanatory
variables. The effect is presented by the hazard ratio similar to the
relative risk concept.. A HR above one indicates an increased intensity or
risk for the
event and a value below a decreased intensity or risk. For example, in our
study is
HR=1.62 for a patient with a stage III ovarian cancer compared to a patient
with a
stage I cancer. This means that the stage III patient has an increased
intensity or risk
of 62% for death compared to the stage I patient. In the analysis it is also
found, that a
patient in the highest level of our proteomic index has a RH=2.64,
corresponding to
an increased intensity or risk of death of 164% compared to a patient with
proteomic
index one unit lower. Corresponding to this indicates a HR above one a poor
prognosis and a HR below one a more favorable prognosis.
A statistical test specifies a null hypothesis which is compared to the
alternative hypothesis based on the probability of the observed outcome. If
the
probability of observing the outcome assuming the null hypothesis is below a
prespecified threshold denoted the level of significance then the null
hypothesis is
rejected in favor of the alternative hypothesis. The probability of
incorrectly rejecting
the null hypothesis, i.e. the null hypothesis is true, is the chosen level of
significance
often denoted the Type I error. A good result is the rejection of the null
hypothesis
when the alternative is true, the probability of this is called the power of
the test and is
- 8 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
dependent on the difference compared to the null hypothesis and the chosen
level of
significance.
Methods of measuring the biomarkers include use of a biochip array. Biochip
arrays useful in the invention include protein and nucleic acid arrays. One or
more
markers are captured on the biochip array and subjected to laser ionization to
detect
the molecular weight of the markers. Analysis of the markers is, for example,
by
molecular weight of the one or more markers against a threshold intensity that
is
normalized against total ion current. Preferably, logarithmic transformation
is used
for reducing peak intensity ranges to limit the number of markers detected.
Another method of measuring the biomarkers includes the use of a
combinatorial ligand library synthesized on beads as described in USSN:
11/495,842,
filed July 28, 2006 and entitled "Methods for Reducing the range in
Concentrations of
Analyte Species in a Sample"; hereby incorporated by reference in its
entirety.
In other methods of the present invention, the step of correlating the
measurement of the biomarkers with ovarian cancer patient survival status is
performed by a software classification algorithm. For example, data is
generated on
subject samples on a biochip array, by subjecting said biochip array to laser
ionization
and detecting intensity of signal for mass/charge ratio; and, transforming the
data into
computer readable form; and executing an algorithm that classifies the data
according
to user input parameters, for detecting signals that represent markers present
in
ovarian cancer patients and are lacking in non-cancer subject controls.
Biochip surfaces are, for example, ionic, anionic, comprised of immobilized
nickel ions, comprised of a mixture of positive and negative ions, comprised
of one or
more antibodies, single or double stranded nucleic acids, proteins, peptides
or
fragments thereof, amino acid probes, or phage display libraries.
In other preferred methods one or more of the markers are measured using
laser desorption/ionization mass spectrometry, comprising providing a probe
adapted
for use with a mass spectrometer comprising an adsorbent attached thereto, and
contacting the subject sample with the adsorbent, and; desorbing and ionizing
the
marker or markers from the probe and detecting the deionized/ionized markers
with
the mass spectrometer.
Preferably, the laser desorption/ionization mass spectrometry comprises:
providing a substrate comprising an adsorbent attached thereto; contacting the
subject
sample with the adsorbent; placing the substrate on a probe adapted for use
with a
- 9 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
mass spectrometer comprising an adsorbent attached thereto; and, desorbing and
ionizing the marker or markers from the probe and detecting the
desorbed/ionized
marker or markers with the mass spectrometer.
The adsorbent can for example be hydrophobic, hydrophilic, ionic or metal
chelate adsorbent, such as, nickel or an antibody, single- or double stranded
oligonucleotide, amino acid, protein, peptide or fragments thereof.
The methods of the present invention can be performed on any type of patient
sample that would be amenable to such methods, e.g., blood, serum and plasma.
The present invention also provides kits comprising capture reagents that bind
the biomarkers and a container comprising the panel of biomarkers. While the
capture reagent can be any type of reagent, preferably the reagent is a SELDI
probe.
In certain kits of the present invention, the capture reagent comprises an
immobilized metal chelate ("IMAC").
Certain kits of the present invention further comprise a wash solution that
selectively allows retention of the bound biomarker to the capture reagent as
compared with other biomarkers after washing.
The invention also provides kits comprising capture reagents that bind the
three biomarkers and instructions for using the capture reagent to measure the
biomarkers. In certain of these kits, the capture reagent comprises an
antibody.
Furthermore, some kits further comprise an MS probe to which the capture
reagent is
attached or is attachable. In some kits, the capture reagent comprises an
IMAC. The
kits may also contain a wash solution that selectively allows retention of the
bound
biomarker to the capture reagent as compared with other biomarkers after
washing.
Preferably, the kit comprises written instructions for use of the kit for
determining
ovarian cancer status and the instructions provide for contacting a test
sample with the
capture reagents and measuring one or more biomarkers retained by the capture
reagents.
The kit also provides for capture reagents, which are antibodies, single or
double stranded oligonucleotide, amino acid, protein, peptide or fragments
thereof.
Measurement of one or more protein biomarkers using the kit, is by mass
spectrometry or immunoassays such as an ELISA.
Purified proteins for detection of ovarian cancer and/or generation of
antibodies for further diagnostic assays are also provided for.
- 10 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
The invention also provides an article manufacture comprising capture
reagents bound to the panel of biomarkers.
Other aspects of the invention are described infra.
BRIEF DESCRIPTION OF THE DRAWINGS
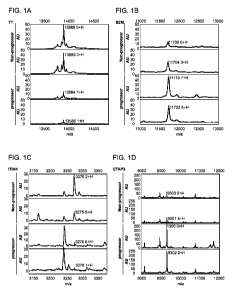
Figures 1A-1D depicts representative spectra from non-progressing OC
patients (top two spectra) and progressing OC patients (bottom two spectra).
A,
transthyretin (TRF); B, beta 2 microglobulin (B2M); C, ITIH4; D, CTAP3.
Figures 2A-2B depicts Kaplan-Meier curves describing the association
between the xb-pro index and A. patients with residual tumor after surgery
(N=92)
and B. all ovarian cancer patients (N=150). Patients were divided into three
groups
using the first and second tertiles of the xb-pro index as cutpoints. For both
patient
groups a highly significant better survival was observed between patients with
xb-pro
index in the upper tertile compared with patients with lower xb-pro index
values.
Figure 3 depicts a plot showing hazard ratios for different combinations of
the
3 intensities, B2M on the abscissae and for 1 and third quartiles of TRF and
ITIH4, all
HR compared to a patient with a median level of each peak.
DEFINITIONS
Unless defined otherwise, all technical and scientific terms used herein have
the meaning commonly understood by a person skilled in the art to which this
invention belongs. The following references provide one of skill with a
general
definition of many of the terms used in this invention: Singleton et al.,
Dictionary of
Microbiology and Molecular Biology (2nd ed. 1994); The Cambridge Dictionary of
Science and Technology (Walker ed., 1988); The Glossary of Genetics, 5th Ed.,
R.
Rieger et al. (eds.), Springer Verlag (1991); and Hale & Marham, The Harper
Collins
Dictionary of Biology (1991). As used herein, the following terms have the
meanings
ascribed to them unless specified otherwise.
"Adsorption" refers to detectable non-covalent binding of an analyte to an
adsorbent or capture reagent.
"Analyte" refers to any component of a sample that is desired to be detected.
The term can refer to a single component or a plurality of components in the
sample.
- 11 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
"Antibody" refers to a polypeptide ligand substantially encoded by an
immunoglobulin gene or immunoglobulin genes, or fragments thereof, which
specifically binds and recognizes an epitope (e.g., an antigen). The
recognized
immunoglobulin genes include the kappa and lambda light chain constant region
genes, the alpha, gamma, delta, epsilon and mu heavy chain constant region
genes,
and the myriad immunoglobulin variable region genes. Antibodies exist, e.g.,
as
intact immunoglobulins or as a number of well-characterized fragments produced
by
digestion with various peptidases. This includes, e.g., Fab' and F(ab)'2
fragments.
The term "antibody," as used herein, also includes antibody fragments either
produced by the modification of whole antibodies or those synthesized de novo
using
recombinant DNA methodologies. It also includes polyclonal antibodies,
monoclonal
antibodies, chimeric antibodies, humanized antibodies, or single chain
antibodies.
"Fe" portion of an antibody refers to that portion of an immunoglobulin heavy
chain
that comprises one or more heavy chain constant region domains, CHi, CH2 and
CH3,
but does not include the heavy chain variable region.
"Biochip" refers to a solid substrate having a generally planar surface to
which
an adsorbent is attached. Frequently, the surface of the biochip comprises a
plurality
of addressable locations, each of which location has the adsorbent bound
there.
Biochips can be adapted to engage a probe interface and, therefore, function
as
probes.
The "complexity" of a sample adsorbed to an adsorption surface of an affinity
capture probe means the number of different protein species that are adsorbed.
The phrase "differentially present" refers to differences in the quantity
and/or
the frequency of a marker present in a sample taken from a subject having or
having a
propensity to develop cancer as compared to a control subject. For example,
the
IAIH4 fragment is present at an elevated level in biological samples obtained
from
ovarian cancer patients as compared to samples from control subjects. In
contrast,
Apo Al and transthyretin described herein are present at a decreased level in
samples
obtained from ovarian cancer patients compared to samples from control
subjects.
Furthermore, a marker can be a polypeptide, which is detected at a higher
frequency
or at a lower frequency in samples of human cancer patients compared to
samples of
control subjects. A marker can be differentially present in terms of level,
quantity,
and/or frequency.
- 12 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
A polypeptide is differentially present between two samples if the
amount/level of the polypeptide in one sample is different from the amount of
the
polypeptide in the other sample. Preferably, the difference is statistically
significant.
For example, a polypeptide is differentially present between the two samples
if it is
present at least about 120%, at least about 130%, at least about 150%, at
least about
180%, at least about 200%, at least about 300%, at least about 500%, at least
about
700%, at least about 900%, or at least about 1000% greater than it is present
in the
other sample, or if it is detectable in one sample and not detectable in the
other.
Alternatively or additionally, a polypeptide is differentially present between
two sets of samples if the frequency of detecting the polypeptide in the
ovarian cancer
patients' samples is statistically significantly higher or lower than in the
control
samples. For example, a polypeptide is differentially present between the two
sets of
samples if it is detected at least about 120%, at least about 130%, at least
about 150%,
at least about 180%, at least about 200%, at least about 300%, at least about
500%, at
least about 700%, at least about 900%, or at least about 1000% more frequently
or
less frequently observed in one set of samples than the other set of samples.
"Diagnostic" means identifying the presence or nature of a pathologic
condition, i.e., ovarian cancer. Diagnostic methods differ in their
sensitivity and
specificity. The "sensitivity" of a diagnostic assay is the percentage of
diseased
individuals who test positive (percent of "true positives"). Diseased
individuals not
detected by the assay are "false negatives." Subjects who are not diseased and
who
test negative in the assay, are termed "true negatives." The "specificity" of
a
diagnostic assay is 1 minus the false positive rate, where the "false
positive" rate is
defined as the proportion of those without the disease who test positive.
While a
particular diagnostic method may not provide a definitive diagnosis of a
condition, it
suffices if the method provides a positive indication that aids in diagnosis.
A "control amount" of a marker can be any amount or a range of amount,
which is to be compared against a test amount of a marker. For example, a
control
amount of a marker can be the amount of a marker in a person without ovarian
cancer.
In one embodiment, a control amount is an absolute amount (e.g., lig/m1). In
another
embodiment, a control amount is the the relative level (e.g., relative
intensity of
signals).
- 13 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
A "diagnostic amount" of a marker refers to an amount of a marker in a
subject's sample that is consistent with a diagnosis of ovarian cancer. In one
embodiment, a diagnostic amount is the absolute amount (e.g., lig/m1) of
analyte. In
another embodiment, a diagnostic amount is the relative level (e.g., relative
intensity
of signals).
"Eluant" or "wash solution" refers to an agent, typically a solution, which is
used to affect or modify adsorption of an analyte to an adsorbent surface
and/or
remove unbound materials from the surface. The elution characteristics of an
eluant
can depend, for example, on pH, ionic strength, hydrophobicity, degree of
chaotropism, detergent strength and temperature.
"Gas phase ion spectrometer" refers to an apparatus that detects gas phase
ions. Gas phase ion spectrometers include an ion source that supplies gas
phase ions.
Gas phase ion spectrometers include, for example, mass spectrometers, ion
mobility
spectrometers, and total ion current measuring devices. "Gas phase ion
spectrometry"
refers to the use of a gas phase ion spectrometer to detect gas phase ions.
"Ion source" refers to a sub-assembly of a gas phase ion spectrometer that
provides gas phase ions. In one embodiment, the ion source provides ions
through a
desorption/ionization process. Such embodiments generally comprise a probe
interface that positionally engages a probe in an interrogatable relationship
to a source
of ionizing energy (e.g., a laser desorption/ionization source) and in
concurrent
communication at atmospheric or subatmospheric pressure with a detector of a
gas
phase ion spectrometer.
Forms of ionizing energy for desorbing/ionizing an analyte from a solid phase
include, for example: (1) laser energy; (2) fast atoms (used in fast atom
bombardment); (3) high energy particles generated via beta decay of
radionucleides
(used in plasma desorption); and (4) primary ions generating secondary ions
(used in
secondary ion mass spectrometry). The preferred form of ionizing energy for
solid
phase analytes is a laser (used in laser desorption/ionization), in
particular, nitrogen
lasers, Nd-Yag lasers and other pulsed laser sources. "Fluence" refers to the
energy
delivered per unit area of interrogated image. A high fluence source, such as
a laser,
will deliver about 1 mJ / mm2 to 50 mJ / mm2. Typically, a sample is placed on
the
surface of a probe, the probe is engaged with the probe interface and the
probe surface
- 14 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
is struck with the ionizing energy. The energy desorbs analyte molecules from
the
surface into the gas phase and ionizes them.
Other forms of ionizing energy for analytes include, for example: (1)
electrons that ionize gas phase neutrals; (2) strong electric field to induce
ionization
from gas phase, solid phase, or liquid phase neutrals; and (3) a source that
applies a
combination of ionization particles or electric fields with neutral chemicals
to induce
chemical ionization of solid phase, gas phase, and liquid phase neutrals.
"Laser desorption mass spectrometer" refers to a mass spectrometer that uses
laser energy as a means to desorb, volatilize, and ionize an analyte.
"Managing subject treatment" refers to the action of a clinician (e.g.,
physician( subsequent to a determination of ovarian cancer status in a
subject. For
example, if the result of the methods of the present invention is inconclusive
or there
is reason that confirmation of status is necessary, the physician may order
more tests.
Alternatively, if the result of the methods of the present invention indicates
a
potentially poor prognosis, alternative or more aggressive therapies may be
warranted.
Furthermore, if the results show a potentially good prognosis, no or less
aggressive
therapies may be warranted.
Examples of more aggressive therapy include: a) The physician may after
surgery treat the patient with more intensive and prolonged chemotherapy. b)
Offer
additional chemotherapy or biological treatments. c) The patient may be
monitored
more closely for relapse or progressive disease. d) Patients with both an
indication of
a poor prognosis and extensive disease, which on imaging indicate nonradical
surgery, may be offered neoadjuvant chemotherapy and subsequent interval
surgery.
e) The proteomic index may be part in the total clinical judgment of treatment
versus
palliative treatment in severe ill patients. f) Radical and correct staged
patients with
stage one and grade 1-2 may be offered adjuvant treatment. g) The patients may
be
selected for surgery by a gynecologic-oncologic surgeon experienced in
performing
extensive procedures. Examples of less aggressive therapy include. a) The
index
may be part of the decision making for radical surgery. b) Radical and correct
staged
patients with stage one and grade 1-2 may avoid a potentially harmful
chemotherapy.
c) The patient may be operated on by a less specialized gynecologist.
A prognostic index may in the future be used to select patients for
individualized treatment (e.g. antibody or molecular based). In one
embodiment, a
protein of the invention is the targets of the therapy.
- 15 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
"Marker" in the context of the present invention refers to a polypeptide that
is
differentially present in a sample taken from a patients having human cancer
as
compared to a reference. In one embodiment, the reference is a comparable
sample
taken from a control subject. A control subject may be a person with a
negative
diagnosis or undetectable cancer, such as a normal or healthy subject. The
term
"biomarker" is used interchangeably with the term "marker."
The term "measuring" means methods which include detecting the presence or
absence of marker(s) in the sample, quantifying the amount of marker(s) in the
sample, and/or qualifying the type of biomarker. Measuring can be accomplished
by
methods known in the art and those further described herein, including but not
limited
to SELDI and immunoassay. Any suitable methods can be used to detect and
measure one or more of the markers described herein. These methods include,
without limitation, mass spectrometry (e.g., laser desorption/ionization mass
spectrometry), fluorescence (e.g. sandwich immunoassay), surface plasmon
resonance, ellipsometry and atomic force microscopy.
"Mass analyzer" refers to a sub-assembly of a mass spectrometer that
comprises a means for measuring a parameter that can be translated into mass-
to-
charge ratios of gas phase ions. In a time-of-flight mass spectrometer the
mass
analyzer comprises an ion optic assembly, a flight tube and an ion detector.
"Mass spectrometer" refers to a gas phase ion spectrometer that measures a
parameter that can be translated into mass-to-charge ratios of gas phase ions.
Mass
spectrometers generally include an ion source and a mass analyzer. Examples of
mass
spectrometers are time-of-flight, magnetic sector, quadrupole filter, ion
trap, ion
cyclotron resonance, electrostatic sector analyzer and hybrids of these. "Mass
spectrometry" refers to the use of a mass spectrometer to detect gas phase
ions.
"Tandem mass spectrometer" refers to any mass spectrometer that is capable
of performing two successive stages of m/z-based discrimination or measurement
of
ions, including ions in an ion mixture. The phrase includes mass spectrometers
having two mass analyzers that are capable of performing two successive stages
of
m/z-based discrimination or measurement of ions tandem-in-space. The phrase
further includes mass spectrometers having a single mass analyzer that is
capable of
performing two successive stages of m/z-based discrimination or measurement of
ions
tandem-in-time. The phrase thus explicitly includes Qq-TOF mass spectrometers,
ion
trap mass spectrometers, ion trap-TOF mass spectrometers, TOF-TOF mass
- 16 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
spectrometers, Fourier transform ion cyclotron resonance mass spectrometers,
electrostatic sector ¨ magnetic sector mass spectrometers, and combinations
thereof.
"Probe" in the context of this invention refers to a device adapted to engage
a
probe interface of a gas phase ion spectrometer (e.g., a mass spectrometer)
and to
present an analyte to ionizing energy for ionization and introduction into a
gas phase
ion spectrometer, such as a mass spectrometer. A "probe" will generally
comprise a
solid substrate (either flexible or rigid) comprising a sample presenting
surface on
which an analyte is presented to the source of ionizing energy.
"Solid support" refers to a solid material which can be derivatized with, or
otherwise attached to, a capture reagent. Exemplary solid supports include
probes,
microtiter plates and chromatographic resins.
"Three biomarker panel" refers to a set of biomarkers identified herein. In
one
embodiment, the three biomarkers are inter-alpha (globulin) inhibitor H4
(plasma
Kallikrein- sensitive glycoprotein) (ITIH4), transferrin (TFR), and beta-2
microglobin
(B2M).
"Surface-enhanced laser desorption/ionization" or "SELDI" refers to a method
of desorption/ionization gas phase ion spectrometry (e.g., mass spectrometry)
in
which the analyte is captured on the surface of a SELDI probe that engages the
probe
interface of the gas phase ion spectrometer. In "SELDI MS," the gas phase ion
spectrometer is a mass spectrometer. SELDI technology is described in, e.g.,
U.S.
patent 5,719,060 (Hutchens and Yip) and U.S. patent 6,225,047 (Hutchens and
Yip).
"Surface-Enhanced Affinity Capture" or "SEAC" is a version of SELDI that
involves the use of probes comprising an absorbent surface (a "SEAC probe").
"Adsorbent surface" refers to a surface to which is bound an adsorbent (also
called a
"capture reagent" or an "affinity reagent"). An adsorbent is any material
capable of
binding an analyte (e.g., a target polypeptide or nucleic acid).
"Chromatographic
adsorbent" refers to a material typically used in chromatography.
Chromatographic
adsorbents include, for example, ion exchange materials, metal chelators
(e.g.,
nitriloacetic acid or iminodiacetic acid), immobilized metal chelates,
hydrophobic
interaction adsorbents, hydrophilic interaction adsorbents, dyes, simple
biomolecules
(e.g., nucleotides, amino acids, simple sugars and fatty acids) and mixed mode
adsorbents (e.g., hydrophobic attraction/electrostatic repulsion adsorbents).
"Biospecific adsorbent" refers an adsorbent comprising a biomolecule, e.g., a
nucleic
acid molecule (e.g., an aptamer), a polypeptide, a polysaccharide, a lipid, a
steroid or
- 17 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
a conjugate of these (e.g., a glycoprotein, a lipoprotein, a glycolipid, a
nucleic acid
(e.g., DNA)-protein conjugate). In certain instances the biospecific adsorbent
can be
a macromolecular structure such as a multiprotein complex, a biological
membrane or
a virus. Examples of biospecific adsorbents are antibodies, receptor proteins
and
nucleic acids. Biospecific adsorbents typically have higher specificity for a
target
analyte than chromatographic adsorbents. Further examples of adsorbents for
use in
SELDI can be found in U.S. Patent 6,225,047 (Hutchens and Yip, "Use of
retentate
chromatography to generate difference maps," May 1, 2001).
In some embodiments, a SEAC probe is provided as a pre-activated surface
which can be modified to provide an adsorbent of choice. For example, certain
probes are provided with a reactive moiety that is capable of binding a
biological
molecule through a covalent bond. Epoxide and carbodiimidizole are useful
reactive
moieties to covalently bind biospecific adsorbents such as antibodies or
cellular
receptors.
"Surface-Enhanced Neat Desorption" or "SEND" is a version of SELDI that
involves the use of probes comprising energy absorbing molecules chemically
bound
to the probe surface. ("SEND probe.") "Energy absorbing molecules" ("EAM")
refer
to molecules that are capable of absorbing energy from a laser desorption/
ionization
source and thereafter contributing to desorption and ionization of analyte
molecules in
contact therewith. The phrase includes molecules used in MALDI , frequently
referred to as "matrix", and explicitly includes cinnamic acid derivatives,
sinapinic
acid ("SPA"), cyano-hydroxy-cinnamic acid ("CHCA") and dihydroxybenzoic acid,
ferulic acid, hydroxyacetophenone derivatives, as well as others. It also
includes
EAMs used in SELDI. SEND is further described in United States patent
5,719,060
and United States patent application 60/408,255, filed September 4, 2002
(Kitagawa,
"Monomers And Polymers Having Energy Absorbing Moieties Of Use In
Desorption/Ionization Of Analytes").
"Surface-Enhanced Photolabile Attachment and Release" or "SEPAR" is a
version of SELDI that involves the use of probes having moieties attached to
the
surface that can covalently bind an analyte, and then release the analyte
through
breaking a photolabile bond in the moiety after exposure to light, e.g., laser
light.
SEPAR is further described in United States patent 5,719,060.
"Molecular binding partners" and "specific binding partners" refer to pairs of
molecules, typically pairs of biomolecules that exhibit specific binding.
Molecular
- 18 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
binding partners include, without limitation, receptor and ligand, antibody
and
antigen, biotin and avidin, and biotin and streptavidin.
"Monitoring" refers to recording changes in a continuously varying parameter.
"Protein biochip" refers to a biochip adapted for the capture of polypeptides.
Many protein biochips are described in the art. These include, for example,
protein
biochips produced by Ciphergen Biosystems (Fremont, CA), Packard BioScience
Company (Meriden CT), Zyomyx (Hayward, CA) and Phylos (Lexington, MA).
Examples of such protein biochips are described in the following patents or
patent
applications: U.S. patent 6,225,047 (Hutchens and Yip, "Use of retentate
chromatography to generate difference maps," May 1, 2001); International
publication WO 99/51773 (Kuimelis and Wagner, "Addressable protein arrays,"
October 14, 1999); U.S. patent 6,329,209 (Wagner et al., "Arrays of protein-
capture
agents and methods of use thereof," December 11, 2001) and International
publication
WO 00/56934 (Englert et al., "Continuous porous matrix arrays," September 28,
2000).
Protein biochips produced by Ciphergen Biosystems comprise surfaces having
chromatographic or biospecific adsorbents attached thereto at addressable
locations.
Ciphergen ProteinChip arrays include NP20, H4, H50, SAX-2, WCX-2, CM-10,
IMAC-3, IMAC-30, LSAX-30, LWCX-30, IMAC-40, PS-10, PS-20 and PG-20.
These protein biochips comprise an aluminum substrate in the form of a strip.
The
surface of the strip is coated with silicon dioxide.
In the case of the NP-20 biochip, silicon oxide functions as a hydrophilic
adsorbent to capture hydrophilic proteins.
H4, H50, SAX-2, WCX-2, CM-10, IIVIAC-3, IMAC-30, PS-10 and PS-20
biochips further comprise a functionalized, cross-linked polymer in the form
of a
hydrogel physically attached to the surface of the biochip or covalently
attached
through a silane to the surface of the biochip. The H4 biochip has isopropyl
functionalities for hydrophobic binding. The H50 biochip has nonylphenoxy-
poly(ethylene glycol)methacrylate for hydrophobic binding. The SAX-2 biochip
has
quaternary ammonium functionalities for anion exchange. The WCX-2 and CM-10
biochips have carboxylate functionalities for cation exchange. The IMAC-3 and
IMAC-30 biochips have nitriloacetic acid functionalities that adsorb
transition metal
ions, such as Cu++ and Ni++, by chelation. These immobilized metal ions allow
adsorption of peptide and proteins by coordinate bonding. The PS-10 biochip
has
- 19 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
carboimidizole functional groups that can react with groups on proteins for
covalent
binding. The PS-20 biochip has epoxide functional groups for covalent binding
with
proteins. The PS-series biochips are useful for binding biospecific
adsorbents, such as
antibodies, receptors, lectins, heparin, Protein A, biotin/streptavidin and
the like, to
chip surfaces where they function to specifically capture analytes from a
sample. The
PG-20 biochip is a PS-20 chip to which Protein G is attached. The LSAX-30
(anion
exchange), LWCX-30 (cation exchange) and IIVIAC-40 (metal chelate) biochips
have
functionalized latex beads on their surfaces. Such biochips are further
described in:
WO 00/66265 (Rich et al., "Probes for a Gas Phase Ion Spectrometer," November
9,
2000); WO 00/67293 (Beecher et al., "Sample Holder with Hydrophobic Coating
for
Gas Phase Mass Spectrometer," November 9, 2000); U.S. patent application
U520030032043A1 (Pohl and Papanu, "Latex Based Adsorbent Chip," July 16, 2002)
and U.S. patent application 60/350,110 (Um et al., "Hydrophobic Surface Chip,"
November 8, 2001).
Upon capture on a biochip, analytes can be detected by a variety of detection
methods selected from, for example, a gas phase ion spectrometry method, an
optical
method, an electrochemical method, atomic force microscopy and a radio
frequency
method. Gas phase ion spectrometry methods are described herein. Of particular
interest is the use of mass spectrometry and, in particular, SELDI. Optical
methods
include, for example, detection of fluorescence, luminescence,
chemiluminescence,
absorbance, reflectance, transmittance, birefringence or refractive index
(e.g., surface
plasmon resonance, ellipsometry, a resonant minor method, a grating coupler
waveguide method or interferometry). Optical methods include microscopy (both
confocal and non-confocal), imaging methods and non-imaging methods.
Immunoassays in various formats (e.g., ELISA) are popular methods for
detection of
analytes captured on a solid phase. Electrochemical methods include voltametry
and
amperometry methods. Radio frequency methods include multipolar resonance
spectroscopy.
A "test amount" of a marker refers to an amount of a marker present in a
sample being tested. A test amount can be either in absolute amount (e.g.,
lig/m1) or a
relative amount (e.g., relative intensity of signals).
DETAILED DESCRIPTION OF THE INVENTION
- 20 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
The present invention provides compositions and methods for determining the
prognosis of subjects having or suspected of having ovarian cancer by
detecting
particular biomarkers. The detection and measurement of these biomarkers in
subject
samples provides information that diagnosticians can correlate with overall
survival
and/or progression-free survival to select an appropriate therapeutic regimen
for the
subject.
The invention is based, at least in part, on the discovery that one or more of
the following biomarkers are useful for detecting and/or characterizing
ovarian cancer
in a subject: apolipoprotein Al (AP0A1), transthyretin (cysteinylated form)
(TT),
inter-alpha trypsin inhibitor IV (internal fragment) (ITIH4), transferrin
(TrF),
hepcidin (HEPC), connective-tissue activating protein 3 (CTAP3), Serum Amyloid
Al (SAA), and beta-2 microglobin (B2M). In particular embodiments, these
biomarkers are used to determine a subject's prognosis (e.g., likely overall
survival
and/or progression free survival). In particular embodiments, the biomarkers
used are
inter-alpha (globulin) inhibitor H4 (plasma Kallikrein- sensitive
glycoprotein) (ITIH4),
transferrin (TFR), and/or beta-2 microglobin (B2M).
These biomarkers have been disclosed in PCT/US2005/010783 (WO
2005/098447); US Patent Application Publication 2005/0059013; PCT/U503/00531
(W003/057014); PCT/U52003/024636 (WO 2004/012588); and PCT/U506/08578,
all of which documents are incorporated herein by reference in their entirety.
These biomarkers assess a patient's survival status after having developed
ovarian cancer and could potentially provide additional information to
physicians for
clinical decision-making. This is supported by Cox multivariate analysis in an
independent validation. For example, several large-scale studies have
suggested that
ovarian cancer patients with surgical procedures operated by gynecological
oncologists tend to have a better long-term survival. However, other studies
concluded that currently only about one third of ovarian cancer patients
undergoing
surgical procedures in the US are treated by gynecological oncologists. With
the
current total number of gynecological oncologists available, it is still not
practical to
have all patients undergoing surgery for suspected ovarian cancer be operated
by
gynecologic oncologists. The biomarkers have the potential to be used to
identify
- 21 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
patients with the lower probability of surviving ovarian cancer and recommend
them
for treatment by gynecologic oncologists.
High-throughput protein profiling combined with effective use of
bioinformatics tools provides a useful approach to screening for cancer
markers.
Briefly, the system used in the present invention utilizes chromatographic
ProteinChip Arrays to assay samples using SELDI (Surface Enhanced Laser
Desorption/Ionization). Proteins bound to the arrays are read in a ProteinChip
Reader, a time-of-flight mass spectrometer.
The present invention is based upon the discovery of protein markers that are
differentially present in samples of ovarian cancer patients and control
subjects, and
the application of this discovery in methods and kits for determining ovarian
cancer
status. These protein markers are found in samples from ovarian cancer
patients at
levels that are different than the levels in samples from women in whom human
cancer is undetectable. Accordingly, the amount of one or more markers found
in a
test sample compared to a control, or the presence or absence of one or more
markers
in the test sample provides useful information regarding the ovarian cancer
status of
the patient.
Due to the dismal prognosis of late stage ovarian cancer, it is the general
consensus that a physician will accept a test with a minimal positive
predictive value
of 10%. Extending this to the general population, a general screening test
would
require a sensitivity greater than 70% and a specificity of 99.6%. Currently,
none of
the existing serologic markers, such as CA125, CA72-4, or M-CSF, individually
delivers such a performance. (Bast, R.C., et al., Int J Biol Markers, 1998;
13:179-87).
The best-characterized tumor marker, CA125, is negative in approximately
30-40% of stage I ovarian carcinomas and its levels are elevated in a variety
of benign
diseases. Its use as a population-based screening tool for early detection and
diagnosis of ovarian cancer is hindered by its low sensitivity and
specificity.
Although pelvic and more recently vaginal sonography has been used to screen
high-
risk patients, neither technique has sufficient sensitivity and/or specificity
to be
applied to the general population. Recent efforts in using CA125 in
combination with
additional tumor markers (Woolas RP XF, et al., J Nall Cancer Inst,
1993;85(21):1748-51; Woolas RP, et al., Gynecol Oncol, 1995;59(1):111-6; Zhang
Z,
- 22 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
et al., Gynecol Oncol, 1999;73(1):56-61; Zhang Z, et al., Use of Multiple
Markers to
Detect Stage I Epithelial Ovarian Cancers: Neural Network Analysis Improves
Performance. American Society of Clinical Oncology 2001; Annual Meeting,
Abstract) in a longitudinal risk of cancer model (Skates SJ, et al., Cancer,
1995;76(10
Suppl):2004-10), and in tandem with ultrasound as a second line test (Jacobs I
DA, et
al., Br Med J, 1993;306(6884):1030-34; Menon U TA, et al., British Journal of
Obstetrics and Gynecology, 2000;107(2):165-69) have shown promising results in
improving overall test specificity, which is critical for a disease such as
ovarian
cancer that has a relatively low prevalence.
DESCRIPTION OF THE BIOMARKERS
ITIH4 FRAGMENTS
Other biomarkers that are useful in the methods of the present invention one
or
more of a closely related set of cleavage fragments of inter-a-trypsin
inhibitor heavy
chain H4 precursor, also referred to alternatively herein as "ITIH4
fragments." ITIH4
fragments are described as biomarkers for ovarian cancer in US patent
publication
2005-0059013 Al, International Patent Publication WO 2005/098447 and Fung et
al.,
Int. J. Cancer 115:783-789 (2005). ITIH4 fragments can be selected from the
group
consisting of ITIH4 fragment no. 1, ITIH4 fragment no. 2, and ITIH4 fragment
no. 3.
The amino acid sequences of the ITIH4 fragments were determined to be:
ITIH4 fragment 1 (SEQ ID NO: 5): MNFRPGVLSSRQLGLPGPPDVPDHAAYHPF
ITIH4 fragment 2 (SEQ ID NO: 6): PGVLSSRQLGLPGPPDVPDHAAYHPF
ITIH4 fragment 3 (SEQ ID NO: 7): GVLSSRQLGLPGPPDVPDHAAYHPF. The
present invention also includes all other known fragments of ITIHA4.
ITIH4 precursor is a 930 amino acid protein (SwissProt Q14624). ITIH4
fragment 1 spans amino acids 658-687 of human ITIH4 precursor. ITIH4 fragment
2
spans amino acids 662-687 of ITIH4 precursor. ITIH4 fragment 3 spans amino
acids
663-687 of ITIH4 precursor.
Additionally, preferred methods of the present invention include the use of
modified forms of ITIH4 fragment. Modification of ITIH4 fragment may include
the
post-translational addition of various chemical groups, for example,
glycosylation,
lipidation, cysteinylation, and glutathionylation.
-23 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
TRANSFERRIN (TRF)
Another biomarker that is useful in the methods of the present invention is
transferrrin. Transferrrin is described as a biomarker for ovarian cancer in
US patent
publication 2005-0214760 Al. Transferrrin is a 679 amino acid protein derived
from
a 698 amino acid precursor (GenBank Accession No. NP_001054 GI:4557871;
SwissProt Accesion No. P02787) (SEQ ID NO: 10). Transferrrin is recognized by
antibodies available from, e.g., Dako (catalog A006) (www.dako.com, Glostrup,
Denmark). Transferrin is glycosylated. Therefore, the measured molecular
weight is
higher than the theoretical weight, which does not take glycosylation into
account.
BETA-2 MICROGLOBIN (B2M)
Another biomarker that is useful in the methods of the present invention is
132-
microglobulin. 132-microglobulin is described as a biomarker for ovarian
cancer in
US provisional patent publication 60/693,679, filed June 24, 2005 (Fung et
al.). 132-
microglobulin is a 99 amino acid protein derived from an 119 amino acid
precursor
(GI:179318; SwissProt Accession No. P61769) (SEQ ID NO: 11). 132-microglobulin
is recognized by antibodies available from, e.g., Abcam (catalog AB759)
(www.abcam.com, Cambridge, MA).
Because, in one embodiment, the biomarkers of this invention are
characterized by mass-to-charge ratio, binding properties and spectral shape,
they can
be detected by mass spectrometry without knowing their specific identity.
However,
if desired, biomarkers whose identity is not determined can be identified by,
for
example, determining the amino acid sequence of the polypeptides. For example,
a
biomarker can be peptide-mapped with a number of enzymes, such as tryp sin or
V8
protease, and the molecular weights of the digestion fragments can be used to
search
databases for sequences that match the molecular weights of the digestion
fragments
generated by the various enzymes. Alternatively, protein biomarkers can be
sequenced using tandem MS technology. In this method, the protein is isolated
by,
for example, gel electrophoresis. A band containing the biomarker is cut out
and the
protein is subject to protease digestion. Individual protein fragments are
separated by
a first mass spectrometer. The fragment is then subjected to collision-induced
cooling, which fragments the peptide and produces a polypeptide ladder. A
- 24 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
polypeptide ladder is then analyzed by the second mass spectrometer of the
tandem
MS. The difference in masses of the members of the polypeptide ladder
identifies the
amino acids in the sequence. An entire protein can be sequenced this way, or a
sequence fragment can be subjected to database mining to find identity
candidates.
U.S. Patent Application No.: 11/373,833, filed March 10, 2006 is hereby
incorporated by reference in its entirety.
It has been found that proteins frequently exist in a sample in a plurality of
different forms characterized by a detectably different mass. These forms can
result
from either, or both, of pre- and post-translational modification. Pre-
translational
modified forms include allelic variants, slice variants and RNA editing forms.
Post-
translationally modified forms include forms resulting from proteolytic
cleavage (e.g.,
fragments of a parent protein), glycosylation, phosphorylation, lipidation,
oxidation,
methylation, cystinylation, sulphonation and acetylation. The collection of
proteins
including a specific protein and all modified forms of it is referred to
herein as a
"protein cluster." The collection of all modified forms of a specific protein,
excluding
the specific protein, itself, is referred to herein as a "modified protein
cluster."
Modified forms of the biomarker of this invention also may be used,
themselves, as
biomarkers. In certain cases the modified forms may exhibit better
discriminatory
power in diagnosis than the specific forms set forth herein.
Modified forms of a biomarker can be initially detected by any methodology
that can detect and distinguish the modified from the biomarker. A preferred
method
for initial detection involves first capturing the biomarker and modified
forms of it,
e.g., with biospecific capture reagents, and then detecting the captured
proteins by
mass spectrometry. More specifically, the proteins are captured using
biospecific
capture reagents, such as antibodies, aptamers or Affibodies that recognize
the
biomarker and modified forms of it. This method also will also result in the
capture
of protein interactors that are bound to the proteins or that are otherwise
recognized
by antibodies and that, themselves, can be biomarkers. In certain embodiments,
the
biospecific capture reagents are bound to a solid phase. Then, the captured
proteins
can be detected by SELDI mass spectrometry or by eluting the proteins from the
capture reagent and detecting the eluted proteins by traditional MALDI or by
SELDI.
- 25 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
The use of mass spectrometry is especially attractive because it can
distinguish and
quantify modified forms of a protein based on mass and without the need for
labeling.
Preferably, the biospecific capture reagent is bound to a solid phase, such as
a
bead, a plate, a membrane or a chip. Methods of coupling biomolecules, such as
antibodies, to a solid phase are well known in the art. They can employ, for
example,
bifunctional linking agents, or the solid phase can be derivatized with a
reactive
group, such as an epoxide or an imidizole, that will bind the molecule on
contact.
Biospecific capture reagents against different target proteins can be mixed in
the same
place, or they can be attached to solid phases in different physical or
addressable
locations. For example, one can load multiple columns with derivatized beads,
each
column able to capture a single protein cluster. Alternatively, one can pack a
single
column with different beads derivatized with capture reagents against a
variety of
protein clusters, thereby capturing all the analytes in a single place.
Accordingly,
antibody-derivatized bead-based technologies, such as xMAP technology of
Luminex
(Austin, TX) can be used to detect the protein clusters. However, the
biospecific
capture reagents must be specifically directed toward the members of a cluster
in
order to differentiate them.
In yet another embodiment, the surfaces of biochips can be derivatized with
the capture reagents directed against protein clusters either in the same
location or in
physically different addressable locations. One advantage of capturing
different
clusters in different addressable locations is that the analysis becomes
simpler.
After identification of modified forms of a protein and correlation with the
clinical parameter of interest, the modified form can be used as a biomarker
in any of
the methods of this invention. At this point, detection of the modified form
can be
accomplished by any specific detection methodology including affinity capture
followed by mass spectrometry, or traditional immunoassay directed
specifically the
modified form. Immunoassay requires biospecific capture reagents, such as
antibodies, to capture the analytes. Furthermore, if the assay must be
designed to
specifically distinguish protein and modified forms of protein. This can be
done, for
example, by employing a sandwich assay in which one antibody captures more
than
one form and second, distinctly labeled antibodies, specifically bind, and
provide
distinct detection of, the various forms. Antibodies can be produced by
immunizing
animals with the biomolecules. This invention contemplates traditional
- 26 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
immunoassays including, for example, sandwich immunoassays including ELISA or
fluorescence-based immunoassays, as well as other enzyme immunoassays.
II. TEST SAMPLES
A) SUBJECT TYPES
Samples are collected from women who have been diagnosed with ovarian
cancer in whom the test is being used to determine their prognosis. Samples
may be
collected from women who had been diagnosed with ovarian cancer and received
treatment to eliminate the cancer, or perhaps are in remission. In a preferred
embodiment, the subjects are women who have been previously diagnosed as
having
ovarian cancer.
B) TYPES OF SAMPLE AND PREPARATION OF THE SAMPLE
The markers can be measured in different types of biological samples. The
sample is preferably a biological fluid sample. Examples of a biological fluid
sample
useful in this invention include blood, blood serum, plasma, vaginal
secretions, urine,
ovarian cyst fluid, tears, saliva, etc. Because all of the markers are found
in blood
serum, blood serum is a preferred sample source for embodiments of the
invention.
If desired, the sample can be prepared to enhance detectability of the
markers.
For example, to increase the detectability of markers, a blood serum sample
from the
subject can be preferably fractionated by, e.g., Cibacron blue agarose
chromatography
and single stranded DNA affinity chromatography, anion exchange
chromatography,
affinity chromatography (e.g., with antibodies) and the like. The method of
fractionation depends on the type of detection method used. Any method that
enriches for the protein of interest can be used. Sample preparations, such as
pre-
fractionation protocols, are optional and may not be necessary to enhance
detectability
of markers depending on the methods of detection used. For example, sample
preparation may be unnecessary if antibodies that specifically bind markers
are used
to detect the presence of markers in a sample.
Typically, sample preparation involves fractionation of the sample and
collection of fractions determined to contain the biomarkers. Methods of pre-
fractionation include, for example, size exclusion chromatography, ion
exchange
chromatography, heparin chromatography, affinity chromatography, sequential
extraction, gel electrophoresis and liquid chromatography. The analytes also
may be
- 27 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
modified prior to detection. These methods are useful to simplify the sample
for
further analysis. For example, it can be useful to remove high abundance
proteins,
such as albumin, from blood before analysis. Examples of methods of
fractionation
are described in PCT/US03/00531 (incorporated herein in its entirety).
Preferably, the sample is pre-fractionated by anion exchange chromatography.
Anion exchange chromatography allows pre-fractionation of the proteins in a
sample
roughly according to their charge characteristics. For example, a Q anion-
exchange
resin can be used (e.g., Q HyperD F, Biosepra), and a sample can be
sequentially
eluted with eluants having different pH's. Anion exchange chromatography
allows
separation of biomolecules in a sample that are more negatively charged from
other
types of biomolecules. Proteins that are eluted with an eluant having a high
pH is
likely to be weakly negatively charged, and a fraction that is eluted with an
eluant
having a low pH is likely to be strongly negatively charged. Thus, in addition
to
reducing complexity of a sample, anion exchange chromatography separates
proteins
according to their binding characteristics.
In preferred embodiments, the serum samples are fractionated via anion
exchange chromatography. Signal suppression of lower abundance proteins by
high
abundance proteins presents a significant challenge to SELDI mass
spectrometry.
Fractionation of a sample reduces the complexity of the constituents of each
fraction.
This method can also be used to attempt to isolate high abundance proteins
into a
fraction, and thereby reduce its signal suppression effect on lower abundance
proteins.
Anion exchange fractionation separates proteins by their isoelectric point
(pI).
Proteins are comprised of amino acids, which are ambivalent-their charge
changes
based on the pH of the environment to which they are exposed. A protein's pI
is the
pH at which the protein has no net charge. A protein assumes a neutral charge
when
the pH of the environment is equivalent to pI of the protein. When the pH
rises above
the pI of the protein, the protein assumes a net negative charge. Similarly,
when the
pH of the environment falls below the pI of the protein, the protein has a net
positive
charge. The serum samples were fractionated according to the protocol set
forth in the
Examples below to obtain the markers described herein.
After capture on anion exchange, proteins were eluted in a series of step
washes at pH 9, pH 7, pH 5, pH 4 and pH 3. A panel of three potential
biomarkers
was discovered by UMSA analysis of profiling data of three fractions (pH
9/flow
through, pH 4, and organic solvent). Two of the peaks were from fraction pH 4
at m/z
- 28 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
of 12828 and 28043, both down-regulated in the cancer group, and the third was
from
fraction pH 9/flow through at m/z of 3272, up-regulated in the cancer group.
All
bound to the immobilized metal affinity chromatography array charged with
copper
ions (IIVIAC3-Cu).
Biomolecules in a sample can also be separated by high-resolution
electrophoresis, e.g., one or two-dimensional gel electrophoresis. A fraction
containing a marker can be isolated and further analyzed by gas phase ion
spectrometry. Preferably, two-dimensional gel electrophoresis is used to
generate
two-dimensional array of spots of biomolecules, including one or more markers.
See,
e.g., Jungblut and Thiede, Mass Spectr. Rev. 16:145-162 (1997).
The two-dimensional gel electrophoresis can be performed using methods
known in the art. See, e.g., Deutscher ed., Methods In Enzymology vol. 182.
Typically, biomolecules in a sample are separated by, e.g., isoelectric
focusing, during
which biomolecules in a sample are separated in a pH gradient until they reach
a spot
where their net charge is zero (i.e., isoelectric point). This first
separation step results
in one-dimensional array of biomolecules. The biomolecules in one-dimensional
array is further separated using a technique generally distinct from that used
in the
first separation step. For example, in the second dimension, biomolecules
separated
by isoelectric focusing are further separated using a polyacrylamide gel, such
as
polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate
(SDS-
PAGE). SDS-PAGE gel allows further separation based on molecular mass of
biomolecules. Typically, two-dimensional gel electrophoresis can separate
chemically different biomolecules in the molecular mass range from 1000-
200,000 Da
within complex mixtures. The pI range of these gels is about 3-10 (wide range
gels).
Biomolecules in the two-dimensional array can be detected using any suitable
methods known in the art. For example, biomolecules in a gel can be labeled or
stained (e.g., Coomassie Blue or silver staining). If gel electrophoresis
generates
spots that correspond to the molecular weight of one or more markers of the
invention, the spot can be further analyzed by gas phase ion spectrometry. For
example, spots can be excised from the gel and analyzed by gas phase ion
spectrometry. Alternatively, the gel containing biomolecules can be
transferred to an
inert membrane by applying an electric field. Then a spot on the membrane that
approximately corresponds to the molecular weight of a marker can be analyzed
by
gas phase ion spectrometry. In gas phase ion spectrometry, the spots can be
analyzed
- 29 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
using any suitable techniques, such as MALDI or SELDI (e.g., using ProteinChip
array) as described herein.
Prior to gas phase ion spectrometry analysis, it may be desirable to cleave
biomolecules in the spot into smaller fragments using cleaving reagents, such
as
proteases (e.g., trypsin). The digestion of biomolecules into small fragments
provides
a mass fingerprint of the biomolecules in the spot, which can be used to
determine the
identity of markers if desired.
High performance liquid chromatography (HPLC) can also be used to separate
a mixture of biomolecules in a sample based on their different physical
properties,
such as polarity, charge and size. HPLC instruments typically consist of a
reservoir of
mobile phase, a pump, an injector, a separation column, and a detector.
Biomolecules
in a sample are separated by injecting an aliquot of the sample onto the
column.
Different biomolecules in the mixture pass through the column at different
rates due
to differences in their partitioning behavior between the mobile liquid phase
and the
stationary phase. A fraction that corresponds to the molecular weight and/or
physical
properties of one or more markers can be collected. The fraction can then be
analyzed
by gas phase ion spectrometry to detect markers. For example, the spots can be
analyzed using either MALDI or SELDI (e.g., using ProteinChip array) as
described
herein.
Optionally, a marker can be modified before analysis to improve its resolution
or to determine its identity. For example, the markers may be subject to
proteolytic
digestion before analysis. Any protease can be used. Proteases, such as
trypsin, that
are likely to cleave the markers into a discrete number of fragments are
particularly
useful. The fragments that result from digestion function as a fingerprint for
the
markers, thereby enabling their detection indirectly. This is particularly
useful where
there are markers with similar molecular masses that might be confused for the
marker in question. Also, proteolytic fragmentation is useful for high
molecular
weight markers because smaller markers are more easily resolved by mass
spectrometry. In another example, biomolecules can be modified to improve
detection resolution. For instance, neuraminidase can be used to remove
terminal
sialic acid residues from glycoproteins to improve binding to an anionic
adsorbent
(e.g., cationic exchange ProteinChip arrays) and to improve detection
resolution. In
another example, the markers can be modified by the attachment of a tag of
particular
- 30 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
molecular weight that specifically bind to molecular markers, further
distinguishing
them. Optionally, after detecting such modified markers, the identity of the
markers
can be further determined by matching the physical and chemical
characteristics of
the modified markers in a protein database (e.g., SwissProt).
III. CAPTURE OF MARKERS
Biomarkers can be captured with capture reagents immobilized to a solid
support, such as any biochip described herein, a multiwell microtiter plate or
a resin.
In particular, the biomarkers of this invention are preferably captured on
SELDI
protein biochips. Capture can be on a chromatographic surface or a biospecific
surface. Any of the SELDI protein biochips comprising reactive surfaces can be
used
to capture and detect the biomarkers of this invention. However, the
biomarkers of
this invention bind well to immobilized metal chelates. The IMAC-3 and IMAC 30
biochips, which nitriloacetic acid functionalities that adsorb transition
metal ions,
such as Cu and Ni', by chelation, are the preferred SELDI biochips for
capturing
the biomarkers of this invention. Any of the SELDI protein biochips comprising
reactive surfaces can be used to capture and detect the biomarkers of this
invention.
These biochips can be derivatized with the antibodies that specifically
capture the
biomarkers, or they can be derivatized with capture reagents, such as protein
A or
protein G that bind immunoglobulins. Then the biomarkers can be captured in
solution using specific antibodies and the captured markers isolated on chip
through
the capture reagent.
In general, a sample containing the biomarkers, such as serum, is placed on
the
active surface of a biochip for a sufficient time to allow binding. Then,
unbound
molecules are washed from the surface using a suitable eluant, such as
phosphate
buffered saline. In general, the more stringent the eluant, the more tightly
the proteins
must be bound to be retained after the wash. The retained protein biomarkers
now
can be detected by appropriate means.
IV. DETECTION AND MEASUREMENT OF MARKERS
Once captured on a substrate, e.g., biochip or antibody, any suitable method
can be used to measure a marker or markers in a sample. For example, markers
can
be detected and/or measured by a variety of detection methods including for
example,
gas phase ion spectrometry methods, optical methods, electrochemical methods,
- 31 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
atomic force microscopy and radio frequency methods. Using these methods, one
or
more markers can be detected.
A) SELDI
One preferred method of detection and/or measurement of the biomarkers uses
mass spectrometry and, in particular, "Surface-enhanced laser
desorption/ionization"
or "SELDI". SELDI refers to a method of desorption/ionization gas phase ion
spectrometry (e.g., mass spectrometry) in which the analyte is captured on the
surface
of a SELDI probe that engages the probe interface. In "SELDI MS," the gas
phase
ion spectrometer is a mass spectrometer. SELDI technology is described in more
detail above.
B) IMMUNOASSAY
In another embodiment, an immunoassay can be used to detect and analyze
markers in a sample. This method comprises: (a) providing an antibody that
specifically binds to a marker; (b) contacting a sample with the antibody; and
(c)
detecting the presence of a complex of the antibody bound to the marker in the
sample.
An immunoassay is an assay that uses an antibody to specifically bind an
antigen (e.g., a marker). The immunoassay is characterized by the use of
specific
binding properties of a particular antibody to isolate, target, and/or
quantify the
antigen. The phrase "specifically (or selectively) binds" to an antibody or
"specifically (or selectively) immunoreactive with," when referring to a
protein or
peptide, refers to a binding reaction that is determinative of the presence of
the protein
in a heterogeneous population of proteins and other biologics. Thus, under
designated
immunoassay conditions, the specified antibodies bind to a particular protein
at least
two times the background and do not substantially bind in a significant amount
to
other proteins present in the sample. Specific binding to an antibody under
such
conditions may require an antibody that is selected for its specificity for a
particular
protein. For example, polyclonal antibodies raised to a marker from specific
species
such as rat, mouse, or human can be selected to obtain only those polyclonal
antibodies that are specifically immunoreactive with that marker and not with
other
proteins, except for polymorphic variants and alleles of the marker. This
selection
- 32-
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
may be achieved by subtracting out antibodies that cross-react with the marker
molecules from other species.
Using the purified markers or their nucleic acid sequences, antibodies that
specifically bind to a marker can be prepared using any suitable methods known
in the
art. See, e.g., Coligan, Current Protocols in Immunology (1991); Harlow &
Lane,
Antibodies: A Laboratory Manual (1988); Goding, Monoclonal Antibodies:
Principles and Practice (2d ed. 1986); and Kohler & Milstein, Nature 256:495-
497
(1975). Such techniques include, but are not limited to, antibody preparation
by
selection of antibodies from libraries of recombinant antibodies in phage or
similar
vectors, as well as preparation of polyclonal and monoclonal antibodies by
immunizing rabbits or mice (see, e.g., Huse et al., Science 246:1275-1281
(1989);
Ward et al., Nature 341:544-546 (1989)). Typically a specific or selective
reaction
will be at least twice background signal or noise and more typically more than
10 to
100 times background.
Generally, a sample obtained from a subject can be contacted with the
antibody that specifically binds the marker. Optionally, the antibody can be
fixed to a
solid support to facilitate washing and subsequent isolation of the complex,
prior to
contacting the antibody with a sample. Examples of solid supports include
glass or
plastic in the form of, e.g., a microtiter plate, a stick, a bead, or a
microbead.
Antibodies can also be attached to a probe substrate or ProteinChip array
described
above. The sample is preferably a biological fluid sample taken from a
subject.
Examples of biological fluid samples include blood, serum, plasma, nipple
aspirate,
urine, tears, saliva etc. In a preferred embodiment, the biological fluid
comprises
blood serum. The sample can be diluted with a suitable eluant before
contacting the
sample to the antibody.
After incubating the sample with antibodies, the mixture is washed and the
antibody-marker complex formed can be detected. This can be accomplished by
incubating the washed mixture with a detection reagent. This detection reagent
may
be, e.g., a second antibody which is labeled with a detectable label.
Exemplary
detectable labels include magnetic beads (e.g., DYNABEADSTm), fluorescent
dyes,
radiolabels, enzymes (e.g., horse radish peroxide, alkaline phosphatase and
others
commonly used in an ELISA), and colorimetric labels such as colloidal gold or
colored glass or plastic beads. Alternatively, the marker in the sample can be
detected
using an indirect assay, wherein, for example, a second, labeled antibody is
used to
- 33 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
detect bound marker-specific antibody, and/or in a competition or inhibition
assay
wherein, for example, a monoclonal antibody which binds to a distinct epitope
of the
marker is incubated simultaneously with the mixture.
Methods for measuring the amount of, or presence of, antibody-marker
complex include, for example, detection of fluorescence, luminescence,
chemiluminescence, absorbance, reflectance, transmittance, birefringence or
refractive index (e.g., surface plasmon resonance, ellipsometry, a resonant
mirror
method, a grating coupler waveguide method or interferometry). Optical methods
include microscopy (both confocal and non-confocal), imaging methods and non-
imaging methods. Electrochemical methods include voltametry and amperometry
methods. Radio frequency methods include multipolar resonance spectroscopy.
Methods for performing these assays are readily known in the art. Useful
assays
include, for example, an enzyme immune assay (ETA) such as enzyme-linked
immunosorbent assay (ELISA), a radioimmune assay (RIA), a Western blot assay,
or
a slot blot assay. These methods are also described in, e.g., Methods in Cell
Biology:
Antibodies in Cell Biology, volume 37 (Asai, ed. 1993); Basic and Clinical
Immunology (Stites & Ten, eds., 7th ed. 1991); and Harlow & Lane, supra.
Throughout the assays, incubation and/or washing steps may be required after
each combination of reagents. Incubation steps can vary from about 5 seconds
to
several hours, preferably from about 5 minutes to about 24 hours. However, the
incubation time will depend upon the assay format, marker, volume of solution,
concentrations and the like. Usually the assays will be carried out at ambient
temperature, although they can be conducted over a range of temperatures, such
as
10 C to 40 C.
Immunoassays can be used to determine presence or absence of a marker in a
sample as well as the quantity of a marker in a sample. The amount of an
antibody-
marker complex can be determined by comparing to a standard. A standard can
be,
e.g., a known compound or another protein known to be present in a sample. As
noted above, the test amount of marker need not be measured in absolute units,
as
long as the unit of measurement can be compared to a control.
The methods for detecting these markers in a sample have many applications.
For example, one or more markers can be measured to aid human cancer diagnosis
or
prognosis. In another example, the methods for detection of the markers can be
used
- 34-
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
to monitor responses in a subject to cancer treatment. In another example, the
methods for detecting markers can be used to assay for and to identify
compounds
that modulate expression of these markers in vivo or in vitro. In a preferred
example,
the biomarkers are used to differentiate between the different stages of tumor
progression, thus aiding in determining appropriate treatment and extent of
metastasis
of the tumor.
C) COMBINATORIAL LIGAND LIBRARY BEADS
Another method of measuring the biomarkers includes the use of a
combinatorial ligand library synthesized on beads as described in USSN:
11/495,842,
filed July 28, 2006 and entitled "Methods for Reducing the range in
Concentrations of
Analyte Species in a Sample"; hereby incorporated by reference in its
entirety.
V. DATA ANALYSIS
When the sample is measured and data is generated the data is then analyzed
by a computer software program. Generally, the software can comprise code that
converts signal from the mass spectrometer into computer readable form. The
software also can include code that applies an algorithm to the analysis of
the signal
to determine whether the signal represents a "peak" in the signal
corresponding to a
marker of this invention, or other useful markers. The software also can
include code
that executes an algorithm that compares signal from a test sample to a
typical signal
characteristic of "normal" and human cancer and determines the closeness of
fit
between the two signals. The software also can include code indicating which
the test
sample is closest to, thereby providing a probable diagnosis.
In preferred methods of the present invention, multiple biomarkers are
measured. The use of multiple biomarkers increases the predictive value of the
test
and provides greater utility in diagnosis, toxicology, patient stratification
and patient
monitoring. The process called "Pattern recognition" detects the patterns
formed by
multiple biomarkers greatly improves the sensitivity and specificity of
clinical
proteomics for predictive medicine. Subtle variations in data from clinical
samples,
e.g., obtained using SELDI, indicate that certain patterns of protein
expression can
- 35 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
predict phenotypes such as the presence or absence of a certain disease, a
particular
stage of cancer progression, or a positive or adverse response to drug
treatments.
Data generation in mass spectrometry begins with the detection of ions by an
ion detector as described above. Ions that strike the detector generate an
electric
potential that is digitized by a high speed time-array recording device that
digitally
captures the analog signal. Ciphergen's ProteinChip system employs an analog-
to-
digital converter (ADC) to accomplish this. The ADC integrates detector output
at
regularly spaced time intervals into time-dependent bins. The time intervals
typically
are one to four nanoseconds long. Furthermore, the time-of-flight spectrum
ultimately analyzed typically does not represent the signal from a single
pulse of
ionizing energy against a sample, but rather the sum of signals from a number
of
pulses. This reduces noise and increases dynamic range. This time-of-flight
data is
then subject to data processing. In Ciphergen's ProteinChip software, data
processing typically includes TOF-to-M/Z transformation, baseline subtraction,
high
frequency noise filtering.
TOF-to-M/Z transformation involves the application of an algorithm that
transforms times-of-flight into mass-to-charge ratio (M/Z). In this step, the
signals
are converted from the time domain to the mass domain. That is, each time-of-
flight
is converted into mass-to-charge ratio, or M/Z. Calibration can be done
internally or
externally. In internal calibration, the sample analyzed contains one or more
analytes
of known M/Z. Signal peaks at times-of-flight representing these massed
analytes are
assigned the known M/Z. Based on these assigned M/Z ratios, parameters are
calculated for a mathematical function that converts times-of-flight to M/Z.
In
external calibration, a function that converts times-of-flight to M/Z, such as
one
created by prior internal calibration, is applied to a time-of-flight spectrum
without
the use of internal calibrants.
Baseline subtraction improves data quantification by eliminating artificial,
reproducible instrument offsets that perturb the spectrum. It involves
calculating a
spectrum baseline using an algorithm that incorporates parameters such as peak
width,
and then subtracting the baseline from the mass spectrum.
High frequency noise signals are eliminated by the application of a smoothing
function. A typical smoothing function applies a moving average function to
each
time-dependent bin. In an improved version, the moving average filter is a
variable
width digital filter in which the bandwidth of the filter varies as a function
of, e.g.,
- 36 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
peak bandwidth, generally becoming broader with increased time-of-flight. See,
e.g.,
WO 00/70648, November 23, 2000 (Gavin et al., "Variable Width Digital Filter
for
Time-of-flight Mass Spectrometry").
Analysis generally involves the identification of peaks in the spectrum that
represent signal from an analyte. Peak selection can, of course, be done by
eye.
However, software is available as part of Ciphergen's ProteinChip software
that can
automate the detection of peaks. In general, this software functions by
identifying
signals having a signal-to-noise ratio above a selected threshold and labeling
the mass
of the peak at the centroid of the peak signal. In one useful application many
spectra
are compared to identify identical peaks present in some selected percentage
of the
mass spectra. One version of this software clusters all peaks appearing in the
various
spectra within a defined mass range, and assigns a mass (M/Z) to all the peaks
that are
near the mid-point of the mass (M/Z) cluster.
Peak data from one or more spectra can be subject to further analysis by, for
example, creating a spreadsheet in which each row represents a particular mass
spectrum, each column represents a peak in the spectra defined by mass, and
each cell
includes the intensity of the peak in that particular spectrum. Various
statistical or
pattern recognition approaches can applied to the data.
In one example, Ciphergen's Biomarker Patterns Tm Software is used to detect
a pattern in the spectra that are generated. The data is classified using a
pattern
recognition process that uses a classification model. In general, the spectra
will
represent samples from at least two different groups for which a
classification
algorithm is sought. For example, the groups can be pathological v. non-
pathological
(e.g., cancer v. non-cancer), drug responder v. drug non-responder, toxic
response v.
non-toxic response, progressor to disease state v. non-progressor to disease
state,
phenotypic condition present v. phenotypic condition absent.
The spectra that are generated in embodiments of the invention can be
classified using a pattern recognition process that uses a classification
model. In some
embodiments, data derived from the spectra (e.g., mass spectra or time-of-
flight
spectra) that are generated using samples such as "known samples" can then be
used
to "train" a classification model. A "known sample" is a sample that is pre-
classified
(e.g., cancer or not cancer). Data derived from the spectra (e.g., mass
spectra or time-
of-flight spectra) that are generated using samples such as "known samples"
can then
be used to "train" a classification model. A "known sample" is a sample that
is pre-
- 37 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
classified. The data that are derived from the spectra and are used to form
the
classification model can be referred to as a "training data set". Once
trained, the
classification model can recognize patterns in data derived from spectra
generated
using unknown samples. The classification model can then be used to classify
the
unknown samples into classes. This can be useful, for example, in predicting
whether
or not a particular biological sample is associated with a certain biological
condition
(e.g., diseased vs. non diseased).
The training data set that is used to form the classification model may
comprise raw data or pre-processed data. In some embodiments, raw data can be
obtained directly from time-of-flight spectra or mass spectra, and then may be
optionally "pre-processed" in any suitable manner. For example, signals above
a
predetermined signal-to-noise ratio can be selected so that a subset of peaks
in a
spectrum is selected, rather than selecting all peaks in a spectrum. In
another
example, a predetermined number of peak "clusters" at a common value (e.g., a
particular time-of-flight value or mass-to-charge ratio value) can be used to
select
peaks. Illustratively, if a peak at a given mass-to-charge ratio is in less
than 50% of
the mass spectra in a group of mass spectra, then the peak at that mass-to-
charge ratio
can be omitted from the training data set. Pre-processing steps such as these
can be
used to reduce the amount of data that is used to train the classification
model.
Classification models can be formed using any suitable statistical
classification (or "learning") method that attempts to segregate bodies of
data into
classes based on objective parameters present in the data. Classification
methods may
be either supervised or unsupervised. Examples of supervised and unsupervised
classification processes are described in Jain, "Statistical Pattern
Recognition: A
Review", IEEE Transactions on Pattern Analysis and Machine Intelligence, Vol.
22,
No. 1, January 2000, which is herein incorporated by reference in its
entirety.
In supervised classification, training data containing examples of known
categories are presented to a learning mechanism, which learns one more sets
of
relationships that define each of the known classes. New data may then be
applied to
the learning mechanism, which then classifies the new data using the learned
relationships. Examples of supervised classification processes include linear
regression processes (e.g., multiple linear regression (MLR), partial least
squares
(PLS) regression and principal components regression (PCR)), binary decision
trees
(e.g., recursive partitioning processes such as CART - classification and
regression
- 38 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
trees), artificial neural networks such as backpropagation networks,
discriminant
analyses (e.g., Bayesian classifier or Fischer analysis), logistic
classifiers, and support
vector classifiers (support vector machines).
A preferred supervised classification method is a recursive partitioning
process. Recursive partitioning processes use recursive partitioning trees to
classify
spectra derived from unknown samples. Further details about recursive
partitioning
processes are provided in U.S. 2002 0138208 Al (Paulse et al., "Method for
analyzing mass spectra," September 26, 2002.
In other embodiments, the classification models that are created can be formed
using unsupervised learning methods. Unsupervised classification attempts to
learn
classifications based on similarities in the training data set, without pre
classifying the
spectra from which the training data set was derived. Unsupervised learning
methods
include cluster analyses. A cluster analysis attempts to divide the data into
"clusters"
or groups that ideally should have members that are very similar to each
other, and
very dissimilar to members of other clusters. Similarity is then measured
using some
distance metric, which measures the distance between data items, and clusters
together data items that are closer to each other. Clustering techniques
include the
MacQueen's K-means algorithm and the Kohonen's Self-Organizing Map algorithm.
Learning algorithms asserted for use in classifying biological information are
described in, for example, WO 01/31580 (Barnhill et al., "Methods and devices
for
identifying patterns in biological systems and methods of use thereof," May 3,
2001);
U.S. 2002/0193950 Al (Gavin et al., "Method or analyzing mass spectra,"
December
19, 2002); U.S. 2003/0004402 Al (Hitt et al., "Process for discriminating
between
biological states based on hidden patterns from biological data," January 2,
2003); and
U.S. Patent No.: 7,113,896 Al (Zhang and Zhang, "Systems and methods for
processing biological expression data" March 20, 2003).
More specifically, to obtain the biomarkers, the peak intensity data of
samples
from cancer patients and healthy controls were used as a "discovery set." This
data
were combined and randomly divided into a training set and a test set to
construct and
test multivariate predictive models.
Generally, the data generated from Section IV above is inputted into a
diagnostic algorithm (i.e., classification algorithm as described above). The
classification algorithm is then generated based on the learning algorithm.
The
process involves developing an algorithm that can generate the classification
- 39 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
algorithm. The methods of the present invention generate a more accurate
classification algorithm by accessing a number of ovarian cancer and normal
samples
of a sufficient number based on statistical sample calculations. The samples
are used
as a training set of data on learning algorithm.
The generation of the classification, i.e., diagnostic, algorithm is dependent
upon the assay protocol used to analyze samples and generate the data obtained
in
Section IV above. It is imperative that the protocol for the detection and/or
measurement of the markers (e.g., in step IV) must be the same as that used to
obtain
the data used for developing the classification algorithm. The assay
conditions, which
must be maintained throughout the training and classification systems include
chip
type and mass spectrometer parameters, as well as general protocols for sample
preparation and testing. If the protocol for the detection and/or measurement
of the
markers (step IV) is changed, the learning algorithm and classification
algorithm must
also change. Similarly, if the learning algorithm and classification algorithm
change,
then the protocol for the detection and/or measurement of markers (step IV)
must also
change to be consistent with that used to generate classification algorithm.
Development of a new classification model would require accessing a sufficient
number of ovarian cancer and normal samples, developing a new training set of
data
based on a new detection protocol, generating a new classification algorithm
using the
data and finally, verifying the classification algorithm with a multi-site
study.
The classification models can be formed on and used on any suitable digital
computer. Suitable digital computers include micro, mini, or large computers
using
any standard or specialized operating system such as a Unix, WindowsTM or
LinuxTM
based operating system. The digital computer that is used may be physically
separate
from the mass spectrometer that is used to create the spectra of interest, or
it may be
coupled to the mass spectrometer. If it is separate from the mass
spectrometer, the
data must be inputted into the computer by some other means, whether manually
or
automated.
The training data set and the classification models according to embodiments
of the invention can be embodied by computer code that is executed or used by
a
digital computer. The computer code can be stored on any suitable computer
readable
media including optical or magnetic disks, sticks, tapes, etc., and can be
written in any
suitable computer programming language including C, C++, visual basic, etc.
- 40 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
VI. VARIOUS EMBODIMENTS.
In one embodiment, a serum sample is collected from a patient and then
fractionated using an anion exchange resin as described above. In one
embodiment,
the biomarkers in the sample are captured using an IMAC copper ProteinChip
array.
The markers can then be detected using SELDI. In such a test one can detect
inter-
alpha (globulin) inhibitor H4 (plasma Kallikrein- sensitive glycoprotein)
(ITIH4),
transferrin (TFR), and beta-2 microglobin (B2M). The results are then entered
into a
computer system, which contains an algorithm that is designed using the same
parameters that were used in the learning algorithm and classification
algorithm to
originally determine the biomarkers. The algorithm produces a diagnosis based
upon
the data received relating to each biomarker. For example, the algorithm can
determine the chances of progression free survival (PFS) or overall survival
(OS).
For example, the diagnosis is determined by examining the data produced
from the SELDI tests with the classification algorithm that is developed using
the
biomarkers. The classification algorithm depends on the particulars of the
test
protocol used to detect the biomarkers. These particulars include, for
example,
sample preparation, chip type, mass spectrometer parameters and/or immunoassay
conditions. If the test parameters change, the algorithm must change.
Similarly, if the
algorithm changes, the test protocol must change.
In yet other embodiments, the markers are captured and tested using non-
SELDI formats. In one example, the sample is collected from the patient. The
biomarkers are captured on a substrate using other known means, e.g.,
antibodies to
the markers. The markers are detected using methods known in the art, e.g.,
optical
methods and refractive index. Examples of optical methods include detection of
fluorescence, e.g., ELISA. Examples of refractive index include surface
plasmon
resonance. The results for the markers are then subjected to an algorithm,
which may
or may not require artificial intelligence. The algorithm produces a diagnosis
based
upon the data received relating to each biomarker.
In any of the above methods, the data from the sample may be fed directly
from the detection means into a computer containing the diagnostic algorithm.
Alternatively, the data obtained can be fed manually, or via an automated
means, into
a separate computer that contains the diagnostic algorithm.
- 41 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
VII. DIAGNOSIS OF SUBJECT AND DETERMINATION OF OVARIAN
CANCER SURVIVAL STATUS
This panel of biomarkers comparing inter-alpha (globulin) inhibitor H4
(plasma Kallikrein-sensitive glycoprotein) (ITIH4), transferrin (TFR), and
beta-2
microglobin (B2M), is useful in aiding in the determination of ovarian cancer
status.
First, the selected biomarkesr are measured in a subject sample using the
methods
described herein, e.g., capture on a SELDI biochip followed by detection by
mass
spectrometry. Then, the measurements is compared with a reference amount or
control that allows for determination of the subject's prognosis. The test
amounts as
compared with the prognostic amount thus indicates ovarian cancer prognosis.
While individual biomarkers are useful diagnostic markers, it has been found
that the particular combination of biomarkers provides herein provides
surprisingly
greater predictive value than single markers alone or other combinations of
markers
previously disclosed in the art. Specifically, the detection of this panel of
markers in
a sample increases the percentage of true positive and true negative diagnoses
and
would decrease the percentage of false positive or false negative diagnoses.
Thus,
methods of the present invention comprise the measurement of more than one
biomarker.
The correlation may take into account the amount of the marker or markers in
the sample compared to a control amount of the marker or markers (up or down
regulation of the marker or markers) (e.g., in normal subjects in whom human
cancer
is undetectable). A control can be, e.g., the average or median amount of
marker
present in comparable samples of subjects in which their prognosis is known.
The
control amount is measured under the same or substantially similar
experimental
conditions as in measuring the test amount.
In certain embodiments of the methods of qualifying ovarian cancer status, the
methods further comprise managing subject treatment based on the status. As
aforesaid, such management describes the actions of the physician or clinician
subsequent to determining ovarian cancer status. For example, if the result of
the
methods of the present invention is inconclusive or there is reason that
confirmation
of status is necessary, the physician may order more tests. Alternatively, if
the status
indicates that surgery is appropriate, the physician may schedule the patient
for
surgery. In other instances, the patient may receive chemotherapy either in
lieu of, or
in addition to, surgery. Likewise, if the result is negative, e.g., the status
indicates late
- 42 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
stage ovarian cancer or if the status is otherwise acute, no further action
may be
warranted. Furthermore, if the results show that treatment has been
successful, no
further management may be necessary.
The invention also provides for such methods where the biomarkers (or
specific combination of biomarkers) are measured again after subject
management. In
these cases, the methods are used to monitor the status of the cancer, e.g.,
response to
cancer treatment, remission of the disease or progression of the disease.
Because of
the ease of use of the methods and the lack of invasiveness of the methods,
the
methods can be repeated after each treatment the patient receives. This allows
the
physician to follow the effectiveness of the course of treatment. If the
results show
that the treatment is not effective, the course of treatment can be altered
accordingly.
This enables the physician to be flexible in the treatment options.
In another example, the methods for detecting markers can be used to assay
for and to identify compounds that modulate expression of these markers in
vivo or in
vitro.
VIII. KITS
In yet another aspect, the present invention provides kits for qualifying
ovarian
cancer status, e.g., for determining the prognosis of a subject, wherein the
kits can be
used to measure the markers of the present invention. For example, the kits
can be
used to measure the panel of markers described herein, which are useful in
determining the prognosis of a subject with ovarian cancer. The kits can also
be used
to monitor the patient's response to a course of treatment, enabling the
physician to
modify the treatment based upon the results of the test. In another example,
the kits
can be used to identify compounds that modulate expression of one or more of
the
markers in in vitro or in vivo animal models for ovarian cancer.
The present invention therefore provides kits comprising (a) a capture reagent
that binds the panel of three biomarkers; and (b) a container comprising at
least one of
the biomarkers. The capture reagents may also bind at least one known
biomarker,
Marker 4, e.g., CA125.
While the capture reagents can be any type of reagent, preferably the reagent
is a SELDI probe. In certain kits of the present invention, the capture
reagent
comprises an IMAC. In other embodiments, the reagent is an antibody.
-43 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
Certain kits of the present invention further comprise a wash solution, or
eluant, that selectively allows retention of the bound biomarkers to the
capture
reagents as compared with other biomarkers after washing. Alternatively, the
kit may
contain instructions for making a wash solution, wherein the combination of
the
adsorbent and the wash solution allows detection of the markers using gas
phase ion
spectrometry.
Preferably, the kit comprises written instructions for use of the kit for
detection of the three biomarkers set forth herein and the instructions
provide for
contacting a test sample with the capture reagents and detecting the panel of
biomarkers retained by the capture reagents. For example, the kit may have
standard
instructions informing a consumer how to wash the capture reagents (e.g.,
probes)
after a sample of blood serum contacts the capture reagents. In another
example, the
kit may have instructions for pre-fractionating a sample to reduce complexity
of
proteins in the sample. In another example, the kit may have instructions for
automating the fractionation or other processes.
Such kits can be prepared from the materials described above, and the
previous discussion of these materials (e.g., probe substrates, capture
reagents,
adsorbents, washing solutions, etc.) is fully applicable to this section and
will not be
repeated.
In another embodiment, a kit comprises (a) antibodies that specifically bind
to
the panel of biomarkers; and (b) a detection reagent. Such kits can be
prepared from
the materials described above, and the previous discussion regarding the
materials
(e.g., antibodies, detection reagents, immobilized supports, etc.) is fully
applicable to
this section and will not be repeated. Optionally, the kit may further
comprise pre-
fractionation spin columns. In some embodiments, the kit may further comprise
instructions for suitable operation parameters in the form of a label or a
separate
insert.
Optionally, the kit may further comprise a standard or control information so
that the test sample can be compared with the control information standard to
determine if the test amount of a marker detected in a sample is a diagnostic
amount
consistent with a good or bad prognosis for a subject having ovarian cancer.
The invention also provides an article manufacture comprising at least one
capture reagent bound to the panel of biomarkers provided herein. Examples of
articles of manufacture of the present invention include, but are not limited
to,
- 44 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
ProteinChip Arrays, probes, microtitre plates, beads, test tubes, microtubes,
and any
other solid phase onto which a capture reagent can be incorporated.
The following examples are offered by way of illustration, not by way of
limitation. While specific examples have been provided, the above description
is
illustrative and not restrictive. Any one or more of the features of the
previously
described embodiments can be combined in any manner with one or more features
of
any other embodiments in the present invention. Furthermore, many variations
of the
invention will become apparent to those skilled in the art upon review of the
specification. The scope of the invention should, therefore, be determined not
with
reference to the above description, but instead should be determined with
reference to
the appended claims along with their full scope of equivalents.
All publications and patent documents cited in this application are
incorporated by reference in their entirety for all purposes to the same
extent as if
each individual publication or patent document were so individually denoted.
By
their citation of various references in this document, Applicants do not admit
any
particular reference is "prior art" to their invention.
EXAMPLES
Example 1: Proteomic techniques provide insights into human ovarian cancer
subjects prognosis
Epithelial ovarian cancer (OC) is one of the leading causes of gynaecological
cancer death worldwide. From the nationwide Danish Gynecologic Cancer Database
(DGCD) it is known that on average 470 new OC cases and 140 Low Malignant
Potential (LMP) ovarian tumors appear each year in Denmark [1]. From the DGCD
it
has been shown that the 3-year overall survival stage I-TV OC patients is 53%.
For
stage III OC patients the overall survival is 41%, much lower than the 3-year
overall
survival of 89% for stage I OC patients [1]. Because DGCD was initiated in
2005,
only 3-year stage related survivals are available.
The relatively asymptomatic nature of early stage disease and the lack of
adequate screening tests are the main reasons why more than 70% of cases
present
with late-stage disease (International Federation of Gynecology and Obstetrics
(FIGO) stage III or stage IV). The 5-year overall survival for women diagnosed
with
-45 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
late-stage disease is less than 20%, whereas the corresponding 5-year survival
for
women with early-stage disease (FIGO stage I and II) is approximately 90% [2,
3].
Recent studies have concluded that OC patients treated by gynecologic
oncologists
have better outcomes than patients treated by general gynecologists or general
surgeons [4-7]. Therefore, the choice of debulking rate can be considered one
prognostic factor.
The traditional clinicopathological variables of prognosis in OC, such as
stage,
histological grade, residual tumor and age, although highly useful, still have
limitations in predicting the outcome of individual patients due to disease
heterogeneity [8-11]. Therefore, additional and better factors indicative for
overall
and progression-free survival are needed.
A large number of new potential biological and cytotoxic treatments of OC
have recently emerged. These new treatment modalities have resulted in an
overwhelming interest in predictive and prognostic markers that can
individualize OC
treatment. Although many predictive factors have been found in OC, no reliable
method for selecting patients for individualized treatments has been described
so far.
Clearly, the need for useful prognostic factors in order to optimize treatment
of the
patients diagnosed with OC has to be emphasized.
Proteomic approaches may provide new insights into biomarker discovery and
application. Techniques such as surface-enhanced laser desorption/ionization
time of
flight-mass spectrometry (SELDI-TOF-MS, SELDI) have the potential to measure
large number of proteins in a single sample [12]. Petricoin et al. [13]
discovered
patterns of proteins found in the blood of OC patients, and reported 100%
sensitivity
and 95% specificity for the investigated set of serum samples. Unfortunately,
other
OC data with the same level of sensitivity and specificity have not been
reported [14].
Zhang et al. [15] used a multivariable model to combine apolipoprotein Al
(AP0A1),
transthyretin (cysteinylated form) (TT) and inter-alpha trypsin inhibitor IV
(internal
fragment) (ITIH4) values from 503 patients. Analysed in combination with serum
CA125, the markers had a sensitivity of 74% and a specificity of 94% for
detecting
OC, which was an improvement over CA125 alone. A large-scale multi-centre
study
evaluated a set of seven biomarkers (ITIH4, TT, AP0A1, transferrin (TrF),
hepcidin
(HEPC), connective-tissue activating protein 3 (CTAP3) and Serum Amyloid Al
(SAA), for the detection of OC. A total of 607 sera from five studies were
analysed
using SELDI-MS protocols optimized for the seven biomarkers. All seven
- 46 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
biomarkers individually demonstrated statistically significant ability to
discriminate
for differentiation [16]. However, none of these references described the
specific
combination of inter-alpha (globulin) inhibitor H4 (plasma Kallikrein-
sensitive
glycoprotein), transferrin (TFR), and beta-2 microglobin (B2M) for determining
overall survival or progression free survival, which is important in selecting
an
appropriate therapeutic regimen.
In addition to biomarker and therapeutic target discovery, proteomic
techniques will likely provide insights into a patient's prognosis. Since
patients with
gross similarities in their disease burden do not share the same prognosis,
differences
in the tumor microenvironment likely contribute to their disparate outcomes.
To
clarify this, proteomics may provide additional information about potential
confounding variables.
A study was undertaken of a prospective collection of women from the Danish
Pelvic Mass study. All were candidates for surgery because of a suspicious
pelvic
mass. The aims were to determine if the serum proteomic biomarkers (AP0A1, TT,
HEPC, ITIH4, TrF, CTAP3 and B2M (beta-2 microglobulin)), alone or in
combination, might be indicative of overall survival and/or progression-free
survival
for women diagnosed with OC. These seven biomarkers had not been previously
evaluated in the above prognostic aspects of OC.
Patient Collection
Between September 2004 and January 2008, 838 women admitted to the
Gynecologic Clinic, Rigshospitalet, Denmark for surgery because of a pelvic
mass,
were enrolled into the "Pelvic Mass" study. Of these patients 150 were
diagnosed
with OC (Table 1). All eligible patients.? 18 years with the suspicion of a
pelvic
mass were informed both in writing and verbally and were invited after written
consent to participate in the study. Patients were examined with an abdominal
and
vaginal ultrasound and serum CA-125 was analysed. Exclusion criteria were
pregnancy, previous cancer or borderline tumor, no understanding of
information or
cancellation of surgery because of no suspicion of pelvic disease after
further
examinations.
Table 1. Clinicopathological characteristics and biomarker levels in study
subjects (N=150).
- 47 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
AP01 TT HEPC ITIH4 B2M CTAP3 TrF
Peak intensities:
Median 6.97 4.25 7.36 3.32 2.90 2.48 1.15
Range 4.65-8.20 1.66- 6.64- 3.32-8.32 1.59-5.56 0.97- 0.32-
5.69 10.28 4.10 2.21
Tumor stage (FIGO)* 0.039 0.024 0.003 0.76 0.018 0.42
0.001
Histological type of 0.26 0.76 0.41 0.0005 0.0006 0.64
0.78
tumor*
Performance status* 0.013 0.009 0.018 0.91 <0.0001 0.017
<0.0001
Radicality of primary 0.012 0.0004 0.0001 0.12 <0.0001
0.027 <0.0001
surgery*
* p-value, Spearman Correlation Coefficients
A Risk Malignancy Index (RMI) was calculated based on the ultrasound score
(U), the menopausal score (M), and value of serum CA125. Multilocularity (>
bilocular), solid areas, internal papilla, bilaterality, ascites, and
extraovarian tumors
scored one point each. A total of 2 or more points gave U = 3; fewer than 2
points
gave U = 1. Postmenopausal status was defined as more than 1 year of
amenorrhea or
a previous hysterectomy and age > 50. Premenopausal status scored M = 1 and
postmenopausal M = 3. Serum CA125 was entered directly into the equation:
RMI=UxMxCA125. If RMI was >200, positron emission tomography/computed
tomography (PET/CT) was performed and the patient operated by a specialist in
gynaecologic oncology. If RMI was <200 the patient could be operated by a
general
gynaecologist. In this study six patients had a RMI <200 and 144 patients had
a RMI
>200. All 150 patients were operated by a specialist in gynaecologic oncology.
Surgery was performed through a midline incision with the intention of radical
surgery. If necessary extensive surgery was performed in order to achieve
macro-
radical surgery and removal of all PET/CT positive tumors.
All tissue specimens were examined by a pathologist who specialized in
gynecologic cancer. All patients were registered in DGCD, which is a
compulsory
research and quality on-line database. The FIGO stage distribution was 22
stage I
patients, 14 stage II patients, 80 stage III patients and 34 stage IV
patients. A total of
116 patients had serous adenocarcinama, 7 patients had mucinous adenocarcinoma
and 27 patients had tumors of other histological types.
- 48 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
Furthermore, preoperative performance status for each patient was obtained
from DGCD. Score 0: no signs of activity of disease (N=64), score 1: smaller
signs of
activity of disease (N=57), score 2: patient mobile for more than 50% of day
time
(N=27) and score 4: patient stay in bed, not mobile (N=2).
All cases in this study were traced in the Danish Central Population Register
(CPR) and date of death, emigration up to January 8th 2009, whichever came
first,
were registered. In addition, all women were linked to DGCD and information
about
treatment (surgery and chemotherapy) and cause of death was established. At
the end
of follow-up, a total of 62 OC patients had died from OC (median follow-up
time: 11
months, range: 1-39) and 88 patients were still alive (median follow-up time:
40
months, range: 13-52).
After surgery 129 patients were treated with platinum-paclitaxel based
chemotherapy, 1 patient was treated with Cyclophosphamide and 1 patient
received
Adriamycine treatment. A total of 19 patients did not receive chemotherapy (6
patients were FIGO stage IA highly differentiated, 1 patient had a stage IC, 7
patients
were stage IIIC and 5 patients were stage IV). Twelve of the 19 patients were
too sick
to receive chemotherapy treatment.
Furthermore, information regarding progression was obtained from the
oncological patient files. Standard WHO response criteria were used to verify
response. In short, complete remission was defined as disappearance of all
clinical
symptoms and a serum CA125 level below 35 U/ml, - evaluated after completion
of
first-line chemotherapy, or if serum CA125 value had been higher than 35 U/ml
preoperatively. Progression-free survival was calculated from the date of
surgery to
the date of documented disease progression (clinical, ultrasound, CT or
PET/CT)
and/or biochemical) or end of study, which was January 2008. The collection of
progression data is a more time consuming process than collection of survival
information from registries. Progression data is up to one year older than
survival
data. At the end of follow-up, a total of 80 OC patients had no clinical
symptoms of
progression (median progression free survival: 15 months, range: 1-41) and 70
patients had progression (median progression free survival: 4 months, range: 0-
31).
The Danish Ethical Committee approved the protocol according to the rules
used in International Conference on Harmonisation/Good Clinical Practice
(ICH/GCP) recommendations and the Helsinki and Tokyo conventions (KF01-
227/03 and KF01-143/04).
- 49 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
Blood sample Analysis
All blood samples were collected less than two weeks before surgery. The
samples were sent by special car to the laboratory, centrifuged at 2000 g for
10
minutes at room temperature and fractionated into serum aliquots of
approximately
0.5 ml and stored at -80 C on the day of collection. Handling of blood
samples from
sampling to freezing was time stamped and recorded in the database in order to
secure
the time schedule. Aliquots used for the proteomic study were only been thawed
for
the actual study.
CA125
Serum CA125 was measured using a commercially available immuno assay,
the CA125II assay (Kryptor reagents on the BRAHMS Kryptor, Immunodiagnostic
systems, using the TRACE (Time Resolved Amplified Cryptate Emission)
technology, based on non-radioactive transfer of energy. Intra-as say co-
efficient of
variation (CV) was 6.6% (n=60), whereas the inter-assay CV was 6.2% (n=10) at
a
control sample of 30 U/ml
Pro teomic
The measurement of AP0A1, TT, HEPC, ITIH4, B2M, CTAP3 and TrF could
be accomplished in four assays, depending on the optimal ProteinChip array
chemistry each analyte bound to. All sample and reference dilutions, and array
processing steps were automated using a combination of commercially available
automated workstations Tecan MCA-150 Freedom EVO (Tecan, Durham, NC) and
the BioMek 2000 (Beckman Coulter, Fullereton, CA) to prevent errors and
maintain
protocol consistency.
Data Collection and Analysis
After a final 30 minutes of drying, all arrays were processed in a ProteinChip
SELDI System (Enterprise Edition, Bio-Rad Laboratories) using ProteinChip Data
Management software v3Ø Data acquisition settings were optimized for the
individual analytes and to provide the best performance. After all spectra
were
collected, data was archived and then imported into OvaCalc Software v3.1
(Vermillion Inc). This software package performed all calculations for the
assay
- 50 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
performance QC and the quantitative or semi-quantitative determinations of
each of
the seven analytes.
Statistical Analysis
Descriptive statistics are presented by the median and range. The Spearman
rank correlation was used as a measure of association between quantitative
variables.
Tests for independence between categorical variables were done using the chi-
square
test and tests for location for continuous variables were done using the
Wilcoxon rank
sum test. Univariate survival probability curves for overall survival were
performed
on the entire study population (N=150) and on patients with residual tumor
after
surgery (N=92). The levels of the seven proteomic biomarkers and CA125 were
scored by the log(base2) of the actual values. The impact of the proteomic
prognostic
index (xb-pro) on overall survival was estimated using the multivariable Cox
proportional hazards model [17] removing proteomic variables which were not
significant. The proteomic index was then constructed as the linear
combination of
the selected variables using the estimated regression coefficients. The chosen
model
was assessed using cross validation techniques [18]. The index values have
been
standardized by the mean value and standard deviation. Kaplan-Meier estimates
of
survival probabilities were calculated by grouping patients using the index
tertiles as
cutpoints. The equality of strata were tested using the log rank test.
The assumptions of proportionality and linearity were assessed using
Schoenfeld and martingale residuals as well as graphical methods, the
assumptions
were not rejected. Multivariable Cox proportional hazard regression was done
including the proteomic xb-pro index and adjusting for International
Federation of
Gynaecology and Obstetrics' (FIGO) stage (I, II, III and IV), residual tumor
after
primary surgery (yes/no), performance status (1, 2, 3, 4), age at diagnosis
(linear),
histological type of tumor (serous, mucinous, other types), serum CA125 levels
and
chemotherapy (yes/no). The results for each variable are presented by the
hazard ratio
(HR) and their 95% confidence intervals (95% CI). The same analysis was done
for
the endpoint PFS. P-values less than 5% were considered significant. All
statistical
calculations were done using a commercially available software package, SAS
(v9.1,
SAS Institute, Cary, N.C., USA).
- 51 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
Example 2: Description of the seven markers: AP01, TT, HEPC, ITIH4, B2M,
CTAP3 and TrF.
The patients included in the study were slightly older (65 years, range: 30-
87)
than the median age for Danish patients diagnosed with OC (60 year) [1].
Similarly
with respect to stage and histology this study may reflect the group of women
all
treated at a University Hospital. For all OC patients the median peak
intensity was
6.97 for AP0A1 (range: 4.65-8.20), 4.25 for TT (range: 1.66-5.69), 7.36 for
HEPC
(range: 6.64-10.28), 3.32 for ITIH4 (range: 3.32-8.32), 2.90 for B2M (range:
1.53-
5.56), 2.48 for CTAP3 (range: 0.97-4.10) and 1.15 for TrF (range: 0.32-2.21).
The
median serum CA125 level was 558.5 U/ml (range: 6-17275). Representative
spectra
from non-progressing OC patients and from progressing OC patients are shown in
Figures 1A-1D.
A significant positive correlation was found between serum CA125 and peak
intensities of HEPC (r=0.18, p=0.031), B2M (r=0.27, p=0.0009) and CTAP3
(r=0.26,
p=0.001). A significant negative correlation was found between serum CA125
levels
and peak intensities of AP0A1 (r=-0.24, p=0.003), TT (r=-0.36, p<0.0001), TrF
(r=-
0.29, p=0.0003). All markers except ITIH4 correlated with each other
(strongest
correlation observed was 0.61 (absolute value)).
AP0A1, TT, HEPC, B2M and TrF were all associated with FIGO stage
(AP0A1: p=0.039, TT: p=0.024, HEPC: p=0.003, B2M: p=0.018 and TrF: p=0.001,
Wilcoxon rank sum test). The same markers in addition with CTAP3 were
associated
with performance status (AP0A1: p=0.013, TT: p=0.009, HEPC: p=0.018, B2M:
p<0.0001, TrF: p<0.0001, CTAP3: p=0.017, Wilcoxon rank sum test) and residual
tumor after surgery (AP0A1: p=0.012, TT: p=0.0004, HEPC: p=0.0001, B2M:
p<0.0001, Trf: p<0.0001, CTAP3: p=0.027, Wilcoxon rank sum test). ITIH4 and
B2M were associated with histological type of tumor (ITIH4: p=0.0005, B2M:
p=0.0006, Wilcoxon rank sum test) (Table 1).
Serum proteomic xb-pro index and xb-pfs index for overall and
progression-free survival.
Overall survival - A total of 62 out of the 150 OC patients (41%) died during
follow-up (2 in stage I, 4 in stage II, 37 in stage III and 19 in stage IV).
Univariate
- 52 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
analysis including all OC patients and the seven biomarkers as well as CA125
demonstrated a significant association with survival using the Cox
proportional
hazards model for AP0A1, TT, HEPC, B2M, CTAP3, TrF and CA125 whereas
ITIH4 was not significant (Table 2). Kaplan-Meier curves demonstrating the
association between xb-pro index and patients with residual tumor after
surgery
(N=92), divided into three groups using the first and second tertiles of the
xb-pro
index as cutpoints, are shown in Figure 2A. Similarly, the association between
the
xb-pro index and all OC patients (N=150) is shown in Figure 2B. For both
patient
groups a highly significant better survival was observed between patients with
xb-pro
index in the upper tertile compared with patients with lower xb-pro index
values.
Performing a multivariable Cox survival analysis including all seven
proteomic biomarkers in order to select a possible combination of proteomic
markers
forming a potential prognostic index, and with backwards reduction, the
following
biomarkers were included: ITIH4 (HR=0.67, 95% CI: 0.45-0.99,p=0.042), B2M
(HR=3.07, 95% CI: 2.19-4.31,p<0.0001) and TrF (HR=0.13, 95% CI: 0.06-
0.28,p<0.0001), whereas AP0A1 (p=0.32), TT (p=0.41), HEPC (p=0.32), CTAP3
(p=0.18) were not found to have prognostic importance. B2M was the variable
contributing most to the fit. Removing this variable from the analysis, it was
found
that only ITIH4 and TrF were included in the model. Removing TrF resulted in
CTAP3 and HEPC being retained (p=0.003 and p=0.002 respectively). Both
variables
were moderately associated to TRF suggesting that these variables could
replace TrF.
Removing ITIH4 did not result in other variables being included. Cross
validation of
the model demonstrated that the estimates were robust (B2M: HR=3.09, (95%
CI:2.70-3.54); TrF: HR=0.12, (95% CI:0.09-0.17); ITIH4: HR=0.66, (95% CI: 0.56-
0.78)). None of the other variables contributed substantially to the model
fit.
Table 2. Determinants of survival in 150 stage I-IV ovarian cancer patients.
Ovarall survival (OS) Progression-free survival
(PFS)
Covariate HR (95% p-value HR (95% CI) p-value
CI)
-Fiv
FIGO Stage 1.62 (0.53- 0.40 15.88 (2.69- 0.002
vs
- 53 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
I-FII 5.00) 93)
Residual
tumor after No vs yes 8.24 (2.50- 0.0005 0.78 (0.24- 0.67
surgery 27) 2.51)
Histological type Mucinous vs
2.18 (0.49-
*
serous
9.63) 0.17 0.33
0.57 (0.28-
Other types vs
serous 1.70 (0.94- 1.19)
3.07)
Chemotherapy Yes vs no 0.29 (0.14- 0.0006 *
0.58)
Performance >0 vs 0 1.38 (0.71- 0.34 4.20 (1.73- 0.0015
status 2.65) 10.15)
Age Per 10 years 1.46 (1.12- 0.005 1.44 (1.04- 0.027
1.90) 1.98)
Serum CA125 Log base2 1.00 (0.86- 0.97 1.10 (0.90- 0.36
1.17) 1.33)
Linear predictor 2.64(1.81- <0.0001 ** 1.85 (1.17- 0.009
3.84) 2.92)
*HR: Hazard Ratio
Not included in analysis due to very low number of events. **XB-PFS index
A multivariable Cox survival analysis including the xb-pro index (ITIH4,
B2M and TrF) as a linear predictor adjusting for clinical covariates showed
that xb-
pro (p<0.0001, HR=2.50, 95% CI: 1.65-3.79, residual tumor after primary
surgery
(p=0.0005, HR=0.13, 95% CI: 0.04-0.41), age at diagnosis (p=0.01, HR=1.04, 95%
CI: 1.01-1.07) and chemotherapy (p=0.0002, HR=0.22, 95% CI: 0.10-0.49) all are
of
- 54-
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
independent prognostic value. FIGO stage, performance status, histological
type of
tumor and CA125 had no significant independent prognostic ability (Table 2).
The
type III tests show that the largest chi-square was the xb-pro index (18.49),
chemotherapy (13.66), radicality after primary surgery (12.02), age at
diagnosis
(6.39), FIGO stage (4.96), histological type of tumor (4.23), performance
status
(3.02) and CA125 (0.56). The Cox survival analysis including the xb-pro index
and
the clinical covariates with independent prognostic value did not change the
results.
Progression-free survival
A Univariate Cox regression analyses confined to 120 OC patients (80 patients
with no clinical symptoms of progression and 40 patients developing
progression >1
month after primary surgery) presented the Xb-pro index as independent value
of time
to progression (p<0.0001, HR=2.19, 95% CI: 1.50-3.20).
A proteomic predictive index (xb-pfs) was constructed using the regression
coefficients based on B2M (p=0.001, HR=2.82, 95% CI: 1.52-5.23) and CTAP3
(p=0.002, HR=4.09, 95% CI: 1.67-10.07). The other 5 serum proteomic markers
were found of no value to predict progression-free survival. Cross validation
suggested robust estimates of the linear predictor.
A multivariable Cox regression analysis including the proteomic xb-pfs index
as a linear predictor adjusting for clinical covariates showed that xb-pfs
(p=0.017,
HR=1.84, 95% CI: 1.12-3.03). The results are shown in table 2. In a final Cox
analysis, restricted to FIGO stage III patients, including the proteomic index
xb-pfs,
radicality of primary surgery, age and chemotherapy treatment showed proteomic
xb-
pfs of clinical independent predictive value when including radicality of
primary
surgery, age at diagnosis and treatment in the model (xb-pfs: p=0.008,
HR=1.77, 95%
CI: 1.17-2.70, radicality: p=0.02, HR=0.09, 95% CI: 0.01-0.64, age at
diagnosis:
p=0.04, HR=1.04, 95% CI: 1.00-1.08, chemotherapy: p=0.0006, HR=0.18, 95% CI:
0.07-0.48).
Protein expression profiling using proteomics techniques can be used to
discover novel modified forms of proteins and to determine which combinations
of
proteins are most specifically associated with clinical conditions such as
patient
predictive value and prognosis. Because of its high mortality, OC has received
much
attention from proteomics analysis [13-16]. It is hoped that proteomics will
allow the
- 55 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
development of personalized patient therapy and monitoring of disease.
Numerous
markers have proven useful in individual studies. However, few have proven
useful
when applied to other different populations. This is one factor in determining
the
clinical relevance of candidate biomarkers. ApoAl, TT, and TrF are some of the
biomarkers that have been successfully reproduced in other studies [15, 19-
21].
So far no study has evaluated a specific combination or subset of the seven
biomarkers delineated herein for either classification or for determining
impact on
overall and progression-free survival. A seven marker index has been evaluated
for
diagnostic use by Zhang et al., in which 6 of the markers are the same as the
biomarkers investigated in this study. Zhang was able to differentiate between
patients with OC and patients with benign tumors. However, they did not
investigate
the prognostic value of their biomarkers [16]. Unexpectedly, the three
biomarkers
ITIH4, B2M and TrF had significant independent prognostic value both when
tested
individually and in the xb-pro index. The 3 biomarkers found to be of high
prognostic
independent value as an index (xb-pro), have not earlier been investigated in
OC in
this respect, only as single biomarkers for differentiation of benign and
malignant
patients [22]. ITIH4 levels are enhanced in sera from OC patients compared to
serum
levels found in controls [23]. TT and B2M are reported of prognostic value in
patients with Hodgkins disease and stage II colorectal cancer, respectively
[24, 25].
This study indicates that the 3 biomarkers used in a proteomic prognostic
index (xb-
pro) may correlate with cancer. The index based on 3 biomarkers is even
stronger
than FIGO stage and performance status, which is quite unique for a
biochemical
index. Although none of these markers individually is specific for ovarian
cancer,
specificity is relatively unimportant in the narrow setting of determining
prognosis.
Indeed, it would be interesting to determine whether these indices might have
prognostic value in other cancers.
Cross validation of the selection procedure demonstrated that B2M and TrF
were included in more than 98% of the runs and ITIH4 was selected in more than
50% of the runs. Cross validation of the selected model comprising B2M, TrF
and
ITIH4 showed that the estimated hazard ratios were almost the same as those
found in
the final model suggesting robust estimates.
The biomarker B2M has been found predictive in patients with OC [26]. B2M
is included in the proteomic xb-pro index and therefore the effect of this
index on
progression-free survival was analysed. The optimal proteomic index (xb-pfs)
was
- 56 -
CA 02818593 2013-05-21
WO 2012/054824 PCT/US2011/057271
composed of two biomarkers, B2M and CTAP3, with the strongest effect from B2M.
Therefore, these findings support the earlier observation of B2M as a
predictive
independent marker of OC.
Further proteomic studies that elucidate differences in signaling cascades
between these groups of women may enhance the clinician's ability to predict
patients
at highest risk for relapse at the time of diagnosis. This would promote
rational
treatment decisions that could prevent patients with early-stage disease from
undergoing potentially harmful chemotherapy. In conclusion, seven serum
biomarkers were evaluated alone and in combinations. A proteomic index (xb-
pro)
and its potential for predicting the outcome was investigated and a proteomic
index
(xb-pfs) and its potential for predicting progression-free survival for OC
patients also
investigated. The proteomic index had a very strong independent prognostic
value for
overall survival - even stronger than FIGO stage and B2M as reported earlier.
The panel of three biomarkers provides surprisingly accurate predictive
results
of survival independent of the stage of the cancer.
Example 2: Prognostic panels of biomarkers were analyzed for efficacy.
Seven peaks were considered. All calculations have been done on the log
scale (base 2). The chosen panel are: B2M_B, Trf PR and ITH4_D. These 3 have
been validated as described. The p-values to include the others (TT_D, HEPC_D,
APOAl_D and CTAP_D) are 0.66, 0.56, 0.33 and 0.35 (for OS). The following
table
presents univariable analyses of these peaks for Progression Free Survival
(PFS) and
overall survival (OS).
Table 3
PFS OS
Covariate p-value HR 95% CI p-value HR 95% CI
B2M_B 0.21 1.37 0.82-2.26 <0.0001 2.73 1.84-4.04
Trf_PR 0.003 0.26 0.10-0.64 <0.0001 0.14 0.06-0.35
- 57 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
ITIH4_D 0.30 0.78 0.49-1.24 0.054 0.55
0.30-1.01
TT_D 0.17 0.72 0.45-1.15 0.001 0.56
0.39-0.79
HEPC_D 0.19 1.33 0.87-2.04 0.006 1.58
1.14-2.18
APOLD 0.18 0.68 0.39 0.010 0.51 0.30-0.85
CTAP_D 0.006 2.63 1.32-5.24 0.044 1.76
1.02-3.05
All peaks are significant for OS (note that ITIH4_d just over 0.05).
In order to understand the multivariable analysis, the correlations between
these
variables are analyzed (Spearman rank correlations). See Table 4.
10
Table 4:
- 58 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
1.00000 -0.18333 0.01806 -0.29716 0.28793 -0.26707 0.02930
0.0247 0.8264 0.0002 0.0004 0.0010 0.7219
-0.18333 1.00000 0.12736 0.52714 -0.60877 0.49409 -0.46741
0.0247 0.1204 <.0001 <.0001 <.0001 <.0001
0.01806 0.12736 1.00000 0.02468 0.07251 0.15971 -0.03057
0.8264 0.1204 0.7643 0.3779 0.0509 0.7104
-0.29716 0.52714 0.02468 1.00000 -0.43461 0.44210 -0.24724
lTD 0.0002 <.0001 0.7643 <.0001 <.0001
0.0023
JWPCO
0.28793 -0.60877 0.07251 -0.43461 1.00000 -0.37010 0.17513
inegiONNE 0.0004 <.0001 0.3779 <.0001
<.0001 0.0321
APthUj -0.26707 0.49409 0.15971 0.44210 -0.37010
1.00000 -0.34952
A1110::1 0.0010 <.0001 0.0509 <.0001 <.0001
<.0001
0.02930 -0.46741 -0.03057 -0.24724 0.17513 -0.34952
1.00000
CTAPD 0.7219 <.0001 0.7104 0.0023 0.0321
<.0001
Covariates which are highly correlated will result in only one being chosen in
the multivariable analysis.
When the most significant peak is removed from the analysis (B2M_B, OS),
this leads to only Trf PR being retained (HR=0.14, 95% CI: 0.06-0.35,
p<0.0001). In
this model ITIH4_D is not included (p=0.12). The next step is to exclude
Trf_PR
with the following result: HEPC_D (HR=1.59, 95% CI:1.16-2.19,p=0.004) and
ITIH4_D (HR=0.52, 0.27-0.98, p=0.044), now ITIH4_D is again in the model. This
reflects the rather complicated covariance structure. The analysis is now done
without
HEPC_D, and this leads to TT_D being retained (HR=0.56, 95% CI:0.39-
0.79,p=0.001). The next step (after removing TT_D) shows APOAl_D being
included (HR=0.51, 95% CI:0.30-0.85,p=0.01). The final step includes CTAP_D
(HR=1.76, 95% CI: 1.02-3.05, p=0.044, without ITIH4_D (p-value to include
0.06).
These results demonstrate that the data are quite correlated leading to a
predictive
value for almost all variables, however the chosen panel is significantly
better than the
remaining. The roll of ITIH4_D is clearly associated with other peaks. This
was also
confirmed by the cross validation analyses.
Removing the chosen panel from the analysis leads to only TT_D being
retained (HR=0.56, 95% CI:0.39-0.79,p=0.001). The chi-square value is 366.72
for
the model fit, for the best model, the fit statistic is 329.44. The latter is
substantially
better than the first, indicating a much better fit for the best model.
Although almost all peaks contribute information on prognosis, the peaks
B2M_B, Trf PR and ITIH4_d describe the data considerably better than the peaks
not
- 59 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
chosen. Although ITIH4_D significantly improves the fit, its removal still
results in a
model substantially better than those not including B2M_B and Trf_PR. The
results
of the validation procedures demonstrate that the chosen panel (B2M_B, Trf_PR
and
ITH4_D) was robust in this dataset, i.e. none of the other covariates could
reasonably
replace these.
Example 3: A panel including B2M_B, Trf_PR and ITH4_D had prognostic
value
The 3 selected biomarkers are all statistically significant (p<0.05). The
weakest covariate is ITIH4D. In a model including only B2M_B and TRF_PR, the
hazard ratio for TRF_PR is 0.116 which is very similar to the result seen in
the
selected model (HR=0.126) whereas the HR for B2M_B increases to 3.074 with
inclusion of ITIH4_D (versus 2.690 in the model without ITIH4_D). This
suggests
that the effect of B2M_B is mediated by the inclusion of ITIH4D, i.e. becomes
stronger. The internal validation procedures suggested that B2M_B and TRF_PR
are
very robust estimates and that ITIH4 less so but still reasonably strong. See
Figure 3.
CA125 has been included in the univariable and multivariable analyses, please
see the tables. CA125 is not significant in the multivariable setting.
- 60 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
References
1. Nielsen MLS, Hogdall CK. The Danish Gynecologic Cancer Database - A
nationwide
clinical database for ovarian cancer, endometrial cancer and cervical cancer,
year
2006-2007. Lcegeforeningens Forlag (ISSN 19017877) 2008, in press.
2. Lu KH, Patterson AP, Wang L et al. Selection of potential markers for
epithelial ovarian
cancer with gene expression arrays and recursive descent partition analysis.
Clin
Canc Res 2004, 10, 3291-3300.
3. Rapkiewicz AV, Espina V, Petricoin EF, Liotta LA. Biomarkers of ovarian
tumours. Eur J
Cancer 2004, 40, 2604-2612.
4. Soegaard Andersen E, Knudsen A, Svarrer T et al. The results of treatment
of epithelial
ovarian cancer after centralisation of primary surgery. Results from North
Jutland,
Denmark. Gynecol Oncol 2005, 99, 552-556.
5. Earle CC, Schrag D, Neville BA et al. Effect of surgeon specialty on
processes of care and
outcomes for ovarian cancer patients. J Nall Cancer Inst 2006, 98, 172-180.
6. Engelen MJ, Kos HE, Willemse PH et al. Surgery by consultant gynecologic
oncologists
improves survival in patients with ovarian carcinoma. Cancer 2006, 106, 589-
598.
7. Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short-term
survival for
advanced ovarian, tubal, and peritoneal cancer patients operated at teaching
hospitals.
Int J Gynecol Cancer 2006,16 Suppl 1, 11-17.
8. van der Burg ME. Advanced ovarian cancer. Curr Treat Options Oncol 2001, 2,
109-118.
9. Nielsen JS, Jakobsen E, Holund B, Bertelsen K, Jakobsen A. Prognostic
significance of
p53, Her-2, and EGFR overexpression in borderline and epithelial ovarian
cancer. Int
J Gynecol Cancer 2004, 14, 1086-1096.
10. Paramasivam S, Tripcony L, Crandon A et al. Prognostic importance of
preoperative CA-
125 in International Federation of Gynecology and Obstetrics stage I
epithelial
ovarian cancer: an Australian multicenter study. J Clin Oncol 2005, 23, 5938-
5942.
11. Tingulstad S, Skjeldestad FE, Halvorsen TB, Hagen B. Survival and
prognostic factors in
patients with ovarian cancer. Obstet Gynecol 2003, 101, 885-891.
12. Britten RA, Hardy C, Vlahou A, Gregory B, Gin i PS, Drake R.
Identification of
reproducible low mass SELDI protein profiles specific to cisplatin resistance
in
human ovarian cancer cells. Oncol Rep 2005, 14, 1323-1330.
13. Petricoin EF, Ardekani AM, Hitt BA et al. Use of proteomic patterns in
serum to identify
ovarian cancer. Lancet 2002, 359, 572-577.
14. Baggerly KA, Morris JS, Coombes KR. Reproducibility of SELDI-TOF protein
patterns
in serum: comparing datasets from different experiments. Bioinformatics 2004,
20,
777-785.
- 61 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
15. Zhang Z, Bast Jr RC, Yu Y et al. Three biomarkers identified from serum
proteomic
analysis for the detection of early stage ovarian cancer. Cancer Res 2004, 64,
5882-
5890.
16. Zhang Z, Bast Jr RC, Vergote I et al. A large-scale multi-center
independent validation
study of a panel of seven biomarkers for the detection of ovarian cancer. J
Clin Oncol
2006, 24, 5057.
17. Cox DR. Regression models and life tables. J Roy Stat Soc B 1972, 34, 187-
220.
18. Harrel FEJ, Lee KI, Mark DB. Multivariate prognostic models: issues in
developing
models, evaluating assumptions and adequacy, and measuring and reducing
errors.
Stat Med 1996, 15, 361-387.
19. Rai AJ, Zhang Z, Rosenzweig J et al. Proteomic approaches to tumor marker
discovery.
Arch Pathol Lab Med 2002, 126, 1518-1526.
20. Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R.
Characterization of
serum biomarkers for detection of early stage ovarian cancer. Proteomics 2005,
5,
4589-4596.
21. Kozak KR, Amneus MW, Pusey SM et al. Identification of biomarkers for
ovarian cancer
using strong anion-exchange ProteinChips: potential use in diagnosis and
prognosis.
Proc Natl Acad Sci USA 2003, 100, 12343-12348.
22. Zhao Q, Duan W, Wu YM, Qian XH, Deng XH. [Analysis of serum biomarkers of
ovarian epithelial cancers based on 2-DE DIGE and MALDI TOF/TOF[. Zhonghua
Zhong Liu Za Zhi 2008, 30, 754-758.
23. Mohamed E, Abdul-Rahman PS, Doustjalali SR et al. Lectin-based
electrophoretic
analysis of the expression of the 35 kDa inter-alpha-trypsin inhibitor heavy
chain H4
fragment in sera of patients with five different malignancies. Electrophoresis
2008,
29, 2645-2650.
24. Hann HW, Lange B, Stahlhut MW, McGlynn KA. Prognostic importance of serum
transferrin and ferritin in childhood Hodgkins disease. Cancer 1990, 15, 313-
316.
25. Blum C, Graham A, Yousefzadeh M et al. The expression ratio of Map7/B2M is
prognostic for survival in patients with stage II colon cancer. Int j Oncol
2008, 33,
579-584.
26. Hernadi Z, Molnar V, Juhasz B, Polka R, Margitai B. Der pradiktive Wert
der
Tumormarkerkombination CA-125 und Beta-2-Mikroglobulin bei Ovarialkarzinom.
Zentralbl Gynakol 1992, 114, 6-9.
- 62 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
10
30 The present invention has been described in detail, including the
preferred
embodiments thereof. However, it will be appreciated that those skilled in the
art,
upon consideration of the present disclosure, may make modifications and/or
improvements of this invention and still be within the scope and spirit of
this
invention as set forth in the following claims.
- 63 -
CA 02818593 2013-05-21
WO 2012/054824
PCT/US2011/057271
All publications and patent documents cited in this application are
incorporated by reference in their entirety for all purposes to the same
extent as if
each individual publication or patent document were so individually denoted.
By
their citation of various references in this document, Applicants do not admit
any
particular reference is "prior art" to their invention.
- 64 -