Note: Descriptions are shown in the official language in which they were submitted.
CA 02870583 2014-11-14
TITLE: APPARATUS FOR TEACHING GAS PROPERTIES
FIELD OF THE PRESENT INVENTION
The present invention pertains to the field of educational devices, and more
particularly it pertains to an apparatus to visually demonstrate gas
properties.
BACKGROUND OF THE PRESENT INVENTION
The best teaching methods include physical demonstrations of the subject-
matter being taught. When the new knowledge can be seen, heard or
touched, it is easier to assimilate and to be retained by students. If the
demonstration apparatus also stimulates the imagination, the new
knowledge being taught is more effectively absorbed and remembered.
The properties of gases and fluid mechanics in particular are difficult
subjects to teach because gases are invisible and impalpable. Other that hot
air balloons, which are impractical devices for use in a classroom
environment, teaching methods on gas properties are limited to chalk and
blackboard explanations.
It is believed that there is a need in the education system for an apparatus
that can be used to demonstrate gas densities, buoyancy and the diffusion
rates of different gases. It is believed that there is a need for a classroom
or lecture hall size educational device that can be used to visualize the
presence of a gas therein, and to feel the buoyancy force exerted by that gas
on a floating balloon.
1
CA 02870583 2015-09-22
. SUMMARY OF THE PRESENT INVENTION
In the present invention, there is provided an apparatus having a transparent
casing and an oblong toroidal shape in the form of a bucket elevator. The
apparatus is particularly appropriate for visually demonstrating the
buoyancy of balloons filled with helium gas in ambient air and in argon
gas. The apparatus has appropriate dimensions for operation in a classroom
or lecture hall so that students can learn gas properties by visual
experience.
In a first embodiment of the present invention, there is provided an
apparatus for teaching gas properties, comprising: an oblong hollow casing
having first and second vertical compartments separated by a partition and
a clear cylindrical passage around the circumference thereof. A first and
second sheaves are mounted above one another along the partition. A gas
seal is mounted between the sheaves and the partition. A flexible loop, is
mounted around the sheaves and a series of balloons is attached to the
flexible loop at spaced intervals.
The casing has an upper region and a lower region communicating with the
first and second vertical compartments. The first vertical compartment is
openable to ambient air. The second vertical compartment is filled with a
light gas lighter than ambient air. The lower region or the casing is filled
with a heavy gas heavier than ambient air.
The balloons are filled with the light gas, such that the balloons have
buoyancy in the first vertical compartment and a lack of buoyancy in the
second vertical compartment. The heavy gas impedes the diffusion of
ambient air in the second vertical compartment.
2
CA 02870583 2014-11-14
In another aspect of the present invention, the heavy gas is argon and the
light gas is helium.
In yet another aspect of the present invention, there is provided an
installation for repeatedly teaching gas properties. This installation
comprises the aforesaid apparatus and a pressurized helium gas bottle for
replenishing the light-gas compartment when ambient air has diffused into
the light gas and increased the buoyancy of the balloons in the light-gas
compartment.
Because the apparatus has the shape of a bucket elevator, the rotation of the
balloons and the flexible loop around the sheaves excites the imagination
of students. A first reaction by students is that they believe having
discovered a new self-sustained power generator. They try to imagine ways
to make it work continually without slowing down. The apparatus provides
a visible demonstration of gas densities, gas buoyancy, gas diffusion, and
it excites the imagination of students, helping them to understand and to
remember what has been demonstrated by the operation of the apparatus.
This brief summary has been provided so that the nature of the invention
may be understood quickly. A more complete understanding of the
invention can be obtained by reference to the following detailed description
of the preferred embodiment thereof in connection with the attached
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a front elevation view of a transparent apparatus for teaching gas
properties, according to the preferred embodiment of the present invention;
3
CA 02870583 2014-11-14
FIG. 2 is a top view of the preferred apparatus for teaching gas properties;
FIG. 3 is a first elevation cross-section view of the preferred apparatus as
seen along line 3-3 in FIG. 2;
FIG. 4 is a second elevation cross-section view of the preferred apparatus
as seen along line 4-4 in FIG. 3;
FIG. 5 is an enlarged view of the detail circle 5 in FIG. 4;
FIG. 6 is an enlarged view of a preferred balloon attachment;
FIG. 7 is an enlarged view of a portion of the casing showing a slot and
slot cover in an open mode in the lower end of the light-gas
compartment.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
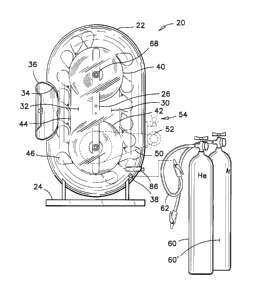
Referring firstly to FIGS. 1 and 2, the apparatus 20 for teaching gas
properties is illustrated therein. Generally, the apparatus 20 has a casing
22 that is made of a transparent material such that the elements mounted
therein and their movements can be observed. The casing 22 is mounted
on a stand 24. The casing 22 is self-supporting on the stand 24. The
apparatus 20 has a relative weight and dimensions such that it can be easily
set and operated in a classroom or lecture hall environment.
The casing 22 has a hollow oblong shape, and is divided in two vertical
=
compartments by a vertical partition 26. The shape of the casing is defined
as a stretched toroidal form defining a clear cylindrical passage around the
4
CA 02870583 2014-11-14
full circumference of the casing 22. The apparatus 20 also has the shape
of a bucket elevator. The longer dimension of the casing 22 is set vertically
on the stand 24.
Referring to FIG. 1, the vertical region on the right-hand side of the
partition 26 is referred to as the light-gas compartment 30, and the vertical
region on the left-hand side of the partition 26 is referred to as the ambient
air compartment 32. The ambient air compartment 32 has an opening 34
in the casing 22, communicating with ambient air outside the apparatus 20.
The casing 22 also has a hinged cover 36 for selectively closing over the
opening 34. The cover 36 preferably has a seal there-around such as to
selectively closing the opening 34 hermetically during the setting-up of the
apparatus 20. The reasons for closing the opening 34 during set-up will be
provided in the following description.
The right-side vertical light-gas compartment 30 has a openable fill plug 38
therein in a lower region thereof for introducing gases to be studied into the
casing 22.
A pair of sheaves 40, 42 are integrated into the partition 26, one above the
other. The sheaves 40, 42 have a same diameter and their centers are set
apart a vertical distance that is greater than that diameter. A flexible loop
44 is threaded around both sheaves 40, 42, and a series of balloons 46 is
attached to the flexible loop 44 at equally spaced intervals along the length
of the flexible loop 44.
The opening 34 and the cover 36 in the ambient air compartment 32 of the
casing 22 are as wide as the casing itself, and at least twice as high as its
width. The opening 34 is wider than the balloons 46 such that the balloons
5
CA 02870583 2014-11-14
46 can be manually attached to, or detached from the flexible loop 44
through this opening 34.
An optional power transmission mechanism 50; a small generator 52; a
light bulb 54 and a switch (not shown) can be mounted to the outside of the
casing 22 to effectually demonstrate the energy available from the
buoyancy of the balloons 46 in different gases during a demonstration
session. These items are optionally used to further excite the imagination
of students being taught gas properties. These items are illustrated in
dashed lines to underline the optional nature of these accessories, and to
show their nonessential aspect in the operation of the apparatus 20.
In FIG. 1, there is also illustrated a pair of pressurized gas bottles 60,
60'.
Preferably, these bottles include a bottle of helium He and a bottle of argon
Ar. A hose and nozzle assembly 62 on each bottle are used to connect
each gas bottle 60, 60' to the fill plug 38 to replenish the amount of gas
required inside the light-gas compartment 30 and in the lower region of the
casing 22, for operating the apparatus.
Referring to FIG. 2, the casing 22 is preferably made of two halves joining
at a seam 66 extending along a median vertical plane of the apparatus 20.
Both halves are held together by bolts 68 extending along the support
bearings of both sheaves 40, 42, for example.
Additional structural details of the preferred apparatus 20 are illustrated in
FIGS. 3, 4, 5, 6 and 7. Both sheaves 40, 42 have smooth surfaces and are
sealed along the partition 26 by a low friction gasket 70. The gas seal or
gasket 70 extends along both sides of the sheaves 40, 42 as well as in the
juxtaposed V-shaped grooves 72 of the sheaves 40, 42, to reduce the
6
CA 02870583 2014-11-14
diffusion of the gases across the partition 26. The vertical space between
the sheaves 40, 42 is blocked by a spacer 74 which supports the gasket 70
sealing the juxtaposed V-shaped grooves 72. The spacer 74 is an integral
part of the partition 26.
Referring to FIG. 6, each balloon 46 is attached to the flexible loop 44, by
a balloon closure 80 and a detachable clip 82 or by any other similar simple
and easy attachment. Preferably, the attachment of the balloons 46 to the
flexible loop 44, is done without difficulty through the ambient air opening
34 in the casing 22.
The expression "flexible loop" 44 is used herein for convenience only. The
expression flexible loop 44 means a flexible band, a line, a thread, a chain,
a rope, a wire, a string, or a belt.
Referring particularly to FIGS. 1 and 3, the operation of the preferred
apparatus 20 will be explained in greater details.
Using a bottle 60 of helium gas He under pressure, all the balloons 46 are
filled with helium gas.
Using the fill plug 38 and a bottle 60' of argon gas Ar, the lower region of
the casing 22 is filled with argon gas Ar up to a desired upper level
enclosing the lower end of the partition 26.
In order to prevent over filling the lower region of the casing 22 with argon
gas Ar, a slot 84 and removable slot cover 86 are provided in the casing 22
at the desired upper level of argon gas in the lower region of the casing 22.
This slot 84 is kept open during the filling of the lower portion of the
7
CA 02870583 2014-11-14
casing with argon gas Ar. The slot 84 and the slot cover 86 are better
illustrated in FIG. 7.
Using the fill plug 38 again, the vertical light-gas compartment 30 is filled
with helium gas He. During the filling up of the light-gas compartment 30
with helium gas He, the slot 84 is also kept open. The ambient air in the
light-gas compartment 30 is forced downward by the rising helium gas He,
pushing the ambient air out of the light-gas compartment 30 through the
slot 84.
During the filling up of the light-gas compartment 30 with helium gas He,
the hinged cover 36 is preferably kept closed on the ambient air opening 34
so that the helium gas He somewhat compresses the ambient air inside the
ambient air compartment 32, which in turn prevents the helium gas He
from escaping out of the casing 22 through the ambient air opening 34.
The slot cover 86 is closed over the slot 84 as soon as the helium gas He
has been introduced in the light-gas compartment 30. Ideally, the helium
gas He fills the light-gas compartment 30 up to about a transition region 90
as shown in FIG. 3.
It will be appreciated that because helium gas He is colourless and
odourless, the filling-up of the light-gas compartment 30 can only be
confirmed by the behaviour of the balloons 46 inside the apparatus 20, and
by the temperature of the casing 22 of the apparatus. Because of the well
known relation of pressure-temperature-volume of gases, the helium gas
entering the light-gas compartment 30 is much colder than ambient air, and
therefore a colder temperature at the upper portion of the casing, at the
transition region 90 for example, is an indication of the presence of helium
8
CA 02870583 2014-11-14
He at that location. Similarly, the temperature of the ambient air exiting the
light-gas compartment 30 through the slot 84, during the filling-up of that
compartment 30 with helium gas He can be monitored in a same way, by
feeling the temperature of the ambient air exiting the slot 84. Therefore,
the temperature of the casing 22, the temperature of the ambient air flowing
out of the slot 84 and the behaviour of the balloons 46 inside the apparatus
20 are three factors providing experience to a user of the apparatus such
that the skills required for setting up the apparatus 20 can be acquired
relatively quickly. Once a time for filling of each of the gases has been
established, a timer (not shown) can be used for the set-up process.
Given that the molecular weights of the three gases present are as
illustrated in Table 1 herein below, the balloons 46 rise in the vertical
ambient air compartment 32, sink on their own weight in the vertical light-
gas compartment 30 and cause the flexible loop 44 and sheaves 40, 42 to
rotate.
Table 1: Gas Molecular weight
Air 26
Argon 39.95
Helium 4.02
The three gases used in the description of the apparatus 20 of the preferred
embodiment are mentioned herein as examples only as other gases can also
be used according to the same principles.
Because the vertical light-gas compartment 30 is sealed at the lower end by
the heavy argon gas Ar, and sealed by the gasket 70 along the partition 26,
9
CA 02870583 2014-11-14
the helium gas He does not escape quickly from the light-gas compartment
30. For a period of time after the light-gas compartment 30 has been filled
with helium gas He, the balloons 46 rise in the ambient air compartment 32
and sink by gravity in the light-gas compartment 30.
A slight resistance is required to pass the balloons 46 through the heavy
argon gas Ar at the bottom of the casing 22, from one vertical compartment
30 to the other 32. However this resistance is less that the torque generated
by the balloons 46 rising in the ambient air compartment 32, and therefore,
the balloons 46 and the flexible loop 44 continue to rotate around both
sheaves 40, 42 for a fair period of time after setup. During this condition,
the effect of gas density on the balloons 46 can be easily observed and
taught to students looking at the apparatus. During this condition, the
generator 52 and light bulb 52 may be used to demonstrate buoyancy forces
associated with the movement of the balloons 46 and the flexible loop 44.
It will be appreciated that the illustrations of the preferred apparatus 20
has
been drawn for dimensional convenience only. This apparatus 20 can be
taller and have many more balloons or other buoyant objects than the
embodiment illustrated in the drawings.
As the balloons 46 rotate around the upper sheave 40, ambient air is carried
by surface tension on the balloons 46 into the light-gas compartment 30.
The helium gas He also seeps slowly through the gasket 70 and into the
ambient air compartment 32. Consequently, the rotation of the balloons 46
around the sheaves 40, 42 eventually stops.
Using the helium gas bottle 60 standing nearby and the fill plug 38, the
light-gas compartment 30 is replenished with helium gas He. As the
CA 02870583 2014-11-14
helium gas He is introduced in the light-gas compartment 30 with the slot
84 open, and replaces the ambient air present there, the balloons 46 and the
flexible loop 44 start to rotate again around the sheaves 40, 42. The
difference in molecular weights between ambient air and helium gas is
thereby convincingly illustrated to students. The balloons 46 "falteringly"
entering the argon gas Ar pool at the lower end of the light-gas
compartment 30 and "popping" out into the lower end of the ambient air
compartment 32 is also a convincing demonstration of the differences in
molecular weights between helium gas and argon gas and between argon
gas and ambient air.
The apparatus for teaching gas properties can also be used to visually
demonstrate the rate of diffusion of one gas into another. This
demonstration is carried out by filling the light-gas compartment 30 with
helium gas He until the concentration of helium gas He in the light-gas
compartment 30 is relatively high, and then letting the apparatus 20 run
until it stops on its own. At that point it will be understood by students
monitoring the operation of the apparatus 20 that ambient air has infiltrated
into the light-gas compartment 30 and that buoyancy on the balloons 46 has
become equal on both sides of the partition 26.
The apparatus 20 can be operated repeatedly until the balloons 46 lose their
helium gas He by permeation. The balloons 46 are easily unhooked from
the flexible loop 44, refilled and reattached to the flexible loop 44 for a
subsequent demonstration to another class of students for example. The
apparatus 20 to teach gas properties according to the preferred embodiment
of the present invention can be used over and over again and is thereby
appropriate for use as a teaching device in a classroom or lecture hall
environment.
11