Note: Descriptions are shown in the official language in which they were submitted.
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
BRIDGED BI-AROMATIC LIGANDS AND OLEFIN POLYMERIZATION
CATALYSTS PREPARED THEREFROM
FIELD
[0001] The present disclosure is directed to bridged bi-aromatic ligands
and transition
metal compounds prepared therefrom. The disclosure is also directed to methods
of preparing
the ligands and transition metal compounds, and to methods of using the
transition metal
compounds as catalyst components in olefin polymerization.
BACKGROUND
[0002] A major focus of the polyolefin industry in recent years has been
on the
development of new catalysts that deliver new and improved products. Bulky
ligand transition
metal compounds, for example, are now widely utilized in catalyst compositions
to produce
polyolefin polymers, such as polyethylene polymers.
[0003] It is recognized in the art that small differences in the
molecular structure of a
catalyst compound can greatly impact catalyst performance and that this is
often governed by
ligand structure. Therefore considerable effort has been expended in designing
new ligand
structures that may lead to catalysts of enhanced performance. WO 03/09162
discloses bridged
bi-aromatic ligands, methods for their preparation and transition metal
compounds derived
therefrom.
[0004] It would be desirable to provide new bridged bi-aromatic ligands
and methods
for their synthesis. It would also be desirable to provide new transition
metal compounds that
can polymerize olefins with useful activity.
SUMMARY
[0005] In one aspect there is provided a bridged bi-aromatic phenol
ligand of formula
(I):
Ri R7
R2 Ar Ar R8
R3 OH OH R9
R4
Ar R6 R12 Ar
R5
R11
(I)
1
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
wherein each of R1, R2, R3, R4, R5, R6, R7, R8, R9, Rio, RH and R12 is
independently selected
from the group consisting of hydride, halide, optionally substituted
hydrocarbyl, heteroatom-
containing optionally substituted hydrocarbyl, alkoxy, aryloxy, silyl, boryl,
dialkyl amino,
alkylthio, arylthio and seleno; optionally two or more R groups can combine
together into ring
structures with such ring structures having from 3 to 100 non-hydrogen atoms
in the ring; A is a
bridging group having from one to 50 non-hydrogen atoms; Y and Y' are
independently selected
from 0, S. NRa and PRa wherein Ra is optionally substituted hydrocarbyl; Ar
is, independently,
optionally substituted aryl or optionally substituted heteroaryl.
[0006]
Each of R1, R2, R3, R4, R5, R6, R7, R8, R9, RI , RH and R12 may be
independently selected from the group consisting of hydride, halide, and
optionally substituted
alkyl, heteroalkyl, aryl, heteroaryl, alkoxyl, aryloxyl, silyl, boryl,
diallcylamino, allcylthio,
arylthio and seleno.
100071
Each of R1, R2, R3, R4, R5, R6, R7, R8, R9, RI , RH and R12 may be
independently selected from the group consisting of hydride, halide, and
optionally substituted
alkyl, heteroalkyl, aryl, heteroaryl, alkoxyl and aryloxyl.
[0008]
Each of R1, R2, R3, R4, R5, R6, R7, R8, R9, RI , RH and R12 may be
independently selected from the group consisting of hydride, fluoro, chloro,
and optionally
substituted alkyl, heteroalkyl, aryl and heteroaryl.
[0009] In
any one of the hereinbefore embodiments the bridging group A may be
selected from the group consisting of optionally substituted divalent
hydrocarbyl and divalent
heteroatom containing hydrocarbyl.
[0010] In
any one of the hereinbefore disclosed embodiments the bridging group A
may be selected from the group consisting of optionally substituted divalent
alkyl, alkenyl,
allcynyl, heteroalkyl, heteroalkenyl, heteroalkynyl, aryl, heteroaryl and
silyl.
[0011] In
any one of the hereinbefore disclosed embodiments the bridging group A
may be represented by the general formula-(QR132_1,,),-wherein each Q is
either carbon or
silicon and each R13 may be the same or different from the others such that
each R13 is selected
from the group consisting of hydride and optionally substituted hydrocarbyl
and heteroatom
containing hydrocarbyl, and optionally two or more R13 groups may be joined
into a ring
structure having from 3 to 50 atoms in the ring structure not counting
hydrogen atoms; z' is an
integer from 1 to 10; and z" is 0, 1 or 2.
[0012] In
any one of the hereinbefore disclosed embodiments Ar may be,
independently, an optionally substituted phenyl, naphthyl, biphenyl,
anthracenyl or
phenanthrenyl.
2
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[0013] In
any one of the hereinbefore disclosed embodiments Ar may be,
independently, an optionally substituted thiophene, pyridine, isoxazole,
pyrazole, pyrrole, furan,
or benzo-fused analogues of these rings.
[0014] In any one of the hereinbefore disclosed embodiments each
occurrence of Ar is
the same.
[0015] The bridged bi-aromatic phenol ligand of formula (I) may be of
formula (II):
Ri
R2 Ar Ar R8
R3 OH OH R9
R4 R10
''''====..A
Ar R6 R12 Ar
R5 R11
(II)
wherein each of RI, R2, R3, R4, R5, R6, R7, R8, R9, RD), R", K-12,
Ar and A is as defined in any
one of the hereinbefore disclosed embodiments.
[0016] In another aspect there is provided a method for preparing a
bridged bi-aromatic
phenol ligand of formula (I) or formula (II) the method comprising at least
one step of
halogenation of an aromatic ring and at least one step of aryl coupling.
[0017] The
method may comprise at least one step of Negishi coupling. The method may
comprise at least one step of Suzuki coupling. The method may comprise both at
least one step
of Negishi coupling and at least one step of Suzuki coupling.
[0018] The method may comprise the steps of:
a) treating a bridged bi-aromatic phenol of formula (III) with a source of
halogen to yield a
tetrahalo bridged bi-aromatic phenol of formula (IV); and
3
84112363
R1 R7
R2 R8
R3 OH OH R9
R4 IIIIJEI
A/Y' Rio
R6 R12
R5 R11
(III)
Ri R7
R2 X X R6
R3 OH OH R9
A/-Y' Rio
X R6 R12 X
R5 Rii
(IV)
b) treating the tetrahalo bridged bi-aromatic phenol of foiniula (IV) with an
aryl-boron
compound (ArBRb2 or ArBF3-10 in the presence of a catalyst, to yield the
bridged
bi-aromatic phenol ligand of formula (I);
wherein each of R1, R2, R3, R4, Rs, R6, R7, R8, R , and X-12
is independently selected
from the group consisting of hydride, halide, optionally substituted
hydrocarbyl, heteroatom-
containing optionally substituted hydrocarbyl, alkoxy, aryloxy, silyl, boryl,
dialkyl amino,
alkylthio, arylthio and seleno; optionally two or more le to R12 groups can
combine together into
ring structures with such ring structures having from 3 to 100 non-hydrogen
atoms in the ring; A
is a bridging group having from one to 50 non-hydrogen atoms; Y and Y' are
independently
selected from 0, S. NW and PR wherein W is optionally substituted hydrocarbyl;
Ar is,
independently, optionally substituted aryl or optionally substituted
heteroaryl; X is halo; Rb is
independently selected from hydride, alkyl, hy droxy and
alkoxy,
4
Date Regue/Date Received 2022-10-17
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
wherein when both of Rb are alkoxy, optionally they may combine to form a ring
structure of
formula BO2Rb2, and wherein 1V1 is an alkali metal cation.
[0019] The method may comprise the steps of:
a) treating a halophenol of formula (V) with a bridged diboron compound of
formula (VI) in the presence of a catalyst to yield the bridged bi-aromatic
phenol
of formula (III);
OH
X
Ri R3
R2
(V)
R4
\A./V R11
R5 R7 R14 R12
R6 R13
(VI)
b) treating the bridged bi-aromatic phenol of formula (III) with a source of
halogen
to yield a tetrahalo bridged bi-aromatic phenol of formula (IV); and
c) treating the tetrahalo bridged bi-aromatic phenol of formula (IV) with an
aryl-
boron compound (ArBRb2 or ArBF3-1\4+) in the presence of a catalyst, to yield
the
bridged bi-aromatic phenol ligand of formula (I);
wherein each of RI, R2, R3, R4, R5, R6, R7, R8, R9, RE), RH_ and R12
is independently selected
from the group consisting of hydride, halide, optionally substituted
hydrocarbyl, heteroatom-
containing optionally substituted hydrocarbyl, alkoxy, aryloxy, silyl, boryl,
dialkyl amino,
arylthio and seleno; optionally two or more R groups can combine together into
ring
structures with such ring structures having from 3 to 100 non-hydrogen atoms
in the ring; A is a
bridging group having from one to 50 non-hydrogen atoms; Y and Y' are
independently selected
from 0, S, NRa and PRa wherein Ra is optionally substituted hydrocarbyl; Z and
Z' are
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
independently selected from BRb2 and BF3-MF, wherein Rb is independently
selected from
hydride, alkyl, hydroxy and alkoxy, wherein when both of Rb are alkoxy,
optionally they may
combine to form a ring structure of formula BO2Rb2, and wherein NI is an
alkali metal cation;
Ar is, independently, optionally substituted aryl or optionally substituted
heteroaryl; X is halo.
[0020] In
any one of the hereinbefore disclosed embodiments each of R1, R2, R3, R4, R5,
R6, R7, R8, R9, RR), RH_ and R12 may
be independently selected from the group consisting of
hydride, halide, and optionally substituted alkyl, heteroalkyl, aryl,
heteroaryl, alkoxyl, aryloxyl,
silyl, boryl, dialkylamino, allcylthio, arylthio and seleno.
[0021] In
any one of the hereinbefore disclosed embodiments each of R1, R2, R3, R4,
R5, R6, R7, R8, R9, RN), R11, and R'2 may be independently selected from the
group consisting of
hydride, halide, and optionally substituted alkyl, heteroallcyl, aryl,
heteroaryl, alkoxyl, aryloxyl,
silyl, diallcylamino, allcylthio, and arylthio.
[0022] In
any one of the hereinbefore disclosed embodiments the bridging group A
may be selected from the group consisting of optionally substituted divalent
hydrocarbyl and
divalent heteroatom containing hydrocarbyl.
[0023] In
any one of the hereinbefore disclosed embodiments the bridging group A
may be selected from the group consisting of optionally substituted divalent
alkyl, alkenyl,
allcynyl, heteroallcyl, heteroalkenyl, heteroalkynyl, carbocycle,
heterocarbocycle, aryl, heteroaryl
and silyl.
[0024] In
any one of the hereinbefore disclosed embodiments the bridging group A
may be represented by the general formula¨(QR132,-)z¨wherein each Q is either
carbon or
silicon and each R13 may be the same or different from the others such that
each R13 is selected
from the group consisting of hydride and optionally substituted hydrocarbyl
and heteroatom
containing hydrocarbyl, and optionally two or more R13 groups may be joined
into a ring
structure having from 3 to 50 atoms in the ring structure not counting
hydrogen atoms; z' is an
integer from 1 to 10; and z" is 0, 1 or 2.
[0025] A
major advantage of the herein disclosed methods is that the number of
reaction steps to access the ligands is low. For example, the disclosed
ligands may be prepared
from a bromophenol in three or four reaction steps.
[0026] In
any one of the hereinbefore disclosed embodiments the catalyst may comprise
a palladium or nickel catalyst.
[0027] In
any one of the hereinbefore disclosed embodiments the palladium catalyst may
comprise a palladium phosphine catalyst.
6
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[0028] In
any one of the hereinbefore disclosed embodiments the catalyst may further
comprise a base.
[0029] In
any one of the hereinbefore disclosed embodiments the base may comprise
an alkali metal carbonate, alkali metal phosphate, alkali metal hydroxide,
alkali metal alkoxide
or an amine.
[0030] In
any one of the hereinbefore disclosed embodiments X may be bromo or chloro.
The source of halogen may be bromine or chlorine.
[0031] In
any one of the hereinbefore disclosed embodiments the aryl-boron
compound may be an optionally substituted arylborane or an optionally
substituted
heteroarylborane.
[0032] In
any one of the hereinbefore disclosed embodiments the aryl-boron compound
may be an optionally substituted aryl boronic acid or an optionally
substituted heteroaryl boronic
acid.
[0033] In
any one of the hereinbefore disclosed embodiments the aryl-boron
compound may be an optionally substituted aryl boronic ester or aryl cyclic
boronic ester or an
optionally substituted heteroaryl boronic ester or hetero aryl cyclic boronic
ester.
[0034] In
any one of the hereinbefore disclosed embodiments the aryl-boron compound
may be an optionally substituted aryl trifluoroborate or an optionally
substituted heteroaryl
trifluoroborate.
[0035] In
another aspect there is provided a ligand of formula (I) or formula (II)
prepared by any one of the hereinbefore disclosed methods.
[0036] In
another aspect there is provided a transition metal compound formed from
any one of the hereinbefore disclosed ligands. The transition metal compound
may comprise a
titanium, a zirconium or a hafnium atom.
[0037] In
another aspect there is provided a catalyst composition comprising one or
more transition metal compounds as hereinbefore disclosed, and one or more
activators. The
activator may comprise one or more alumoxanes. The activator may comprise
methylalumoxane.
[0038] In
another aspect there is provided a supported catalyst composition comprising
one
or more transition metal compounds as hereinbefore disclosed, one or more
activators and one or
more support materials. The activator may comprise one or more alumoxanes. The
activator may
comprise methylalumoxane. The support may be silica.
[0039] The
catalyst composition or supported catalyst composition may comprise two
or more transition metal compounds. The transition metal compounds may be
selected from any
7
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
one of those hereinbefore disclosed or at least one of the transition metal
compounds may be
different from those hereinbefore disclosed. For example, at least one of the
transition metal
compounds may be a metallocene.
[0040] In
another aspect there is provided a process for polymerizing olefins, the
process
comprising:
contacting olefins with one or more catalyst compositions or supported
catalyst compositions
comprising at least one transition metal compound as hereinbefore disclosed in
a reactor under
polymerization conditions to produce an olefin polymer or copolymer
BRIEF DESCRIPTION OF THE DRAWINGS
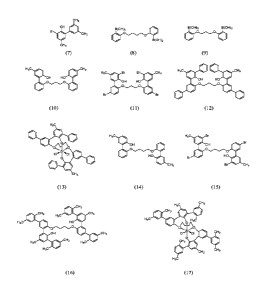
[0041]
Figures 1 and 2 depict the chemical structures of exemplary compounds in
accordance with the present disclosure.
[0042]
Figures 3 to 5 depict exemplary reaction schemes in accordance with the
present
disclosure.
DETAILED DESCRIPTION
[0043]
Before the present compounds, components, compositions, and/or methods are
disclosed and described, it is to be understood that unless otherwise
indicated this invention is
not limited to specific compounds, components, compositions, reactants,
reaction conditions,
ligands, transition metal compounds, or the like, as such may vary, unless
otherwise specified.
It is also to be understood that the terminology used herein is for the
purpose of describing
particular embodiments only and is not intended to be limiting.
[0044] It
must also be noted that, as used in the specification and the appended claims,
the singular forms "a," "an" and "the" include plural referents unless
otherwise specified. Thus,
for example, reference to "a halogen atom" as in a moiety "substituted with a
halogen atom"
includes more than one halogen atom, such that the moiety may be substituted
with two or more
halogen atoms, reference to "a substituent" includes one or more substituents,
reference to "a
ligand" includes one or more ligands, and the like.
[0045] As
used herein, all reference to the Periodic Table of the Elements and groups
thereof is to the NEW NOTATION published in HAWLEY'S CONDENSED CHEMICAL
DICTIONARY, Thirteenth Edition, John Wiley & Sons, Inc., (1997) (reproduced
there with
permission from IUPAC), unless reference is made to the Previous IUPAC form
noted with
Roman numerals (also appearing in the same), or unless otherwise noted.
[0046]
Disclosed herein are ligands, catalyst compounds, catalyst compositions and
supported catalyst compositions for use in the polymerization of olefins which
are advantageous
to prepare and use. Also disclosed herein are methods of making the ligands,
catalyst
8
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
compounds, catalyst compositions and supported catalyst compositions and
polymerization
processes utilizing the catalyst compositions and supported catalyst
compositions for the
production of olefin polymers.
General Definitions
[0047] As used herein, a "catalyst composition" includes one or more
catalyst
compounds utilized to polymerize olefins and at least one activator or,
alternatively, at least one
cocatalyst. The catalyst composition may include any suitable number of
catalyst compounds in
any combination as described herein, as well as any activator or cocatalyst in
any combination as
described herein.
[0048] As used herein, a "supported catalyst composition" includes one or
more
catalyst compounds utilized to polymerize olefins and at least one activator
or, alternatively, at
least one cocatalyst, and at least one support. The supported catalyst
composition may include
any suitable number of catalyst compounds in any combination as described
herein, as well as
any activator or cocatalyst in any combination as described herein. A
"supported catalyst
composition" may also contain one or more additional components known in the
art to reduce or
eliminate reactor fouling such as continuity additives.
[0049] As used herein, a "catalyst compound" may include any compound
that, when
activated, is capable of catalyzing the polymerization or oligomerization of
olefins, wherein the
catalyst compound comprises at least one Group 3 to 12 atom, and optionally at
least one
leaving group bound thereto.
[0050] The term "independently selected" is used herein to indicate that
the R groups,
e.g., RI, R2, R3, ¨4,
K and R5 can be identical or different (e.g. RI, R2, R3, ¨ 4,
K and R5 may all be
substituted alkyls or RI and R2 may be a substituted alkyl and R3 may be an
aryl, etc.). Use of
the singular includes use of the plural and vice versa (e.g., a hexane
solvent, includes hexanes).
A named R group will generally have the structure that is recognized in the
art as corresponding
to R groups having that name. The terms "compound" and "complex" are generally
used
interchangeably in this specification, but those of skill in the art may
recognize certain
compounds as complexes and vice versa. For the purposes of illustration,
representative certain
groups are defined herein. These definitions are intended to supplement and
illustrate, not
preclude, the definitions known to those of skill in the art.
[0051] "Optional" or "optionally" means that the subsequently described
event or
circumstance may or may not occur, and that the description includes instances
where said event
or circumstance occurs and instances where it does not. For example, the
phrase "optionally
substituted hydrocarbyl" means that a hydrocarbyl moiety may or may not be
substituted and
9
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
that the description includes both unsubstituted hydrocarbyl and hydrocarbyl
where there is
substitution.
[0052] The term "alkyl" as used herein refers to a branched or
unbranched saturated
hydrocarbon group typically although not necessarily containing 1 to about 50
carbon atoms,
such as methyl, ethyl, n-propyl, isopropyl, n-butyl, iso-butyl, t-butyl,
octyl, decyl, and the like,
as well as cycloallcyl groups such as cyclopentyl, cyclohexyl and the like.
Generally, although
again not necessarily, alkyl groups herein may contain 1 to about 12 carbon
atoms. The term
"lower alkyl" intends an alkyl group of one to six carbon atoms, specifically
one to four carbon
atoms. "Substituted alkyl" refers to alkyl substituted with one or more
substituent groups (e.g.,
benzyl or chloromethyl), and the terms "heteroatom-containing alkyl" and
"heteroalkyl" refer to
alkyl in which at least one carbon atom is replaced with a heteroatom (e.g., -
CH2OCH3 is an
example of a heteroalkyl).
[0053] The term "alkenyl" as used herein refers to a branched or
unbranched
hydrocarbon group typically although not necessarily containing 2 to about 50
carbon atoms and
at least one double bond, such as ethenyl, n-propenyl, iso-propenyl, n-
butenyl, iso-butenyl,
octenyl, decenyl, and the like. Generally, although again not necessarily,
alkenyl groups herein
contain 2 to about 12 carbon atoms. The term "lower alkenyl" refers to an
alkenyl group of two
to six carbon atoms, specifically two to four carbon atoms. "Substituted
alkenyl" refers to
alkenyl substituted with one or more substituent groups, and the terms
"heteroatom-containing
alkenyl" and "heteroalkenyl" refer to alkenyl in which at least one carbon
atom is replaced with
a heteroatom.
[0054] The term "alkynyl" as used herein refers to a branched or
unbranched
hydrocarbon group typically although not necessarily containing 2 to about 50
carbon atoms and
at least one triple bond, such as ethynyl, n-propynyl, iso-propynyl, n-
butynyl, isobutynyl,
octynyl, decynyl, and the like. Generally, although again not necessarily,
alkynyl groups herein
may have 2 to about 12 carbon atoms. The term "lower alkynyl" refers to an
alkynyl group of
two to six carbon atoms, specifically three or four carbon atoms. "Substituted
alkynyl" refers to
alkynyl substituted with one or more substituent groups, and the terms
"heteroatom-containing
alkynyl" and "heteroalkynyl" refer to alkynyl in which at least one carbon
atom is replaced with
a heteroatom.
[0055] The term "alkoxy" as used herein intends an alkyl group bound
through a
single, terminal ether linkage; that is, an "alkoxy" group may be represented
as -0-alkyl where
alkyl is as defined above. A "lower alkoxy" group refers to an alkoxy group
having one to six,
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
more specifically one to four, carbon atoms. The term "aryloxy" is used in a
similar fashion,
with aryl as defined below. The term "hydroxy" refers to -OH.
[0056] Similarly, the term "allcylthio" as used herein intends an alkyl
group bound
through a single, terminal thioether linkage; that is, an "alkylthio" group
may be represented as -
S-alkyl where alkyl is as defined above. A "lower alkyl thio" group refers to
an alkyl thio group
having one to six, more specifically one to four, carbon atoms. The term
"arylthio" is used
similarly, with aryl as defined below. The term "thioxy" refers to -SH.
[0057] The term "allenyl" is used herein in the conventional sense to
refer to a
molecular segment having the structure -CH=C=CH2. An "allenyl" group may be
unsubstituted
or substituted with one or more non-hydrogen substituents.
[0058] The term "aryl" as used herein, and unless otherwise specified,
refers to an
aromatic substituent containing a single aromatic ring or multiple aromatic
rings that are fused
together, linked covalently, or linked to a common group such as a methylene
or ethylene
moiety. More specific aryl groups contain one aromatic ring or two or three
fused or linked
aromatic rings, e.g., phenyl, naphthyl, biphenyl, anthracenyl, phenanthrenyl,
and the like. The
aryl substituents may have 1 to about 200 carbon atoms, typically 1 to about
50 carbon atoms,
and specifically 1 to about 20 carbon atoms. "Substituted aryl" refers to an
aryl moiety
substituted with one or more substituent groups, (e.g., tolyl, mesityl and
perfluorophenyl) and
the terms "heteroatom-containing aryl" and "heteroaryl" refer to aryl in which
at least one
carbon atom is replaced with a heteroatom (e.g., rings such as thiophene,
pyridine, isoxazole,
pyrazole, pyrrole, furan, etc. or benzo-fused analogues of these rings are
included in the term
"heteroaryl"). In some embodiments herein, multi-ring moieties are
substituents and in such an
embodiment the multi-ring moiety can be attached at an appropriate atom. For
example,
"naphthyl" can be 1-naphthyl or 2-naphthyl; "anthracenyl" can be 1-
anthracenyl, 2-anthracenyl
or 9-anthracenyl; and "phenanthrenyl" can be 1-phenanthrenyl, 2-phenanthrenyl,
3-
phenanthrenyl, 4-phenanthrenyl or 9-phenanthrenyl.
[0059] The term "arallcyl" refers to an alkyl group with an aryl
substituent, and the
term "aralkylene" refers to an alkylene group with an aryl substituent; the
term "alkaryl" refers
to an aryl group that has an alkyl substituent, and the term "alkarylene"
refers to an arylene
group with an alkyl substituent.
[0060] The terms "halo" and "halogen" are used in the conventional sense
to refer to a
chloro, bromo, fluoro or iodo substituent. The terms "haloallcyl,"
"haloalkenyl" or "haloalkynyl"
(or "halogenated alkyl," "halogenated alkenyl," or "halogenated allcynyl")
refers to an alkyl,
11
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
alkenyl or alkynyl group, respectively, in which at least one of the hydrogen
atoms in the group
has been replaced with a halogen atom.
[0061] The term "heteroatom-containing" as in a "heteroatom-containing
hydrocarbyl
group" refers to a molecule or molecular fragment in which one or more carbon
atoms is
replaced with an atom other than carbon, e.g., nitrogen, oxygen, sulfur,
phosphorus, boron or
silicon. Similarly, the term "heteroalkyl" refers to an alkyl substituent that
is heteroatom-
containing, the term "heterocyclic" refers to a cyclic substituent that is
heteroatom-containing,
the term "heteroaryl" refers to an aryl substituent that is heteroatom-
containing, and the like.
When the term "heteroatom-containing" appears prior to a list of possible
heteroatom-containing
groups, it is intended that the term apply to every member of that group. That
is, the phrase
"heteroatom-containing alkyl, alkenyl and alkynyl" is to be interpreted as
"heteroatom-
containing alkyl, heteroatom-containing alkenyl and heteroatom-containing
alkynyl."
100621 "Hydrocarbyl" refers to hydrocarbyl radicals containing 1 to
about 50 carbon
atoms, specifically 1 to about 24 carbon atoms, most specifically 1 to about
16 carbon atoms,
including branched or unbranched, saturated or unsaturated species, such as
alkyl groups,
alkenyl groups, aryl groups, and the like. The term "lower hydrocarbyl" refers
to a hydrocarbyl
group of one to six carbon atoms, specifically one to four carbon atoms.
"Substituted
hydrocarbyl" refers to hydrocarbyl substituted with one or more substituent
groups, and the
terms "heteroatom-containing hydrocarbyl" and "heterohydrocarbyl" refer to
hydrocarbyl in
which at least one carbon atom is replaced with a heteroatom.
[0063] By "substituted" as in "substituted hydrocarbyl," "substituted
aryl," "substituted
alkyl," "substituted alkenyl" and the like, as alluded to in some of the
aforementioned
definitions, is meant that in the hydrocarbyl, hydrocarbylene, alkyl, alkenyl,
aryl or other
moiety, at least one hydrogen atom bound to a carbon atom is replaced with one
or more
substituents that are functional groups such as hydroxyl, alkoxy, alkylthio,
phosphino, amino,
halo, silyl, and the like. When the term "substituted" appears prior to a list
of possible
substituted groups, it is intended that the term apply to every member of that
group. That is, the
phrase "substituted alkyl, alkenyl and alkynyl" is to be interpreted as
"substituted alkyl,
substituted alkenyl and substituted alkynyl." Similarly, "optionally
substituted alkyl, alkenyl and
alkynyl" is to be interpreted as "optionally substituted alkyl, optionally
substituted alkenyl and
optionally substituted alkynyl."
[0064] By "divalent" as in "divalent hydrocarbyl", "divalent alkyl",
"divalent aryl" and
the like, is meant that the hydrocarbyl, alkyl, aryl or other moiety is bonded
at two points to
atoms, molecules or moieties with the two bonding points being covalent bonds.
The term
12
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
"aromatic" is used in its usual sense, including unsaturation that is
essentially delocalized across
multiple bonds, such as around a ring.'
[0065] As
used herein the term "silyl" refers to the -SiZIZ2Z3 radical, where each of
Z1, Z2, and Z3 is independently selected from the group consisting of hydride
and optionally
substituted alkyl, alkenyl, alkynyl, heteroatom-containing alkyl, heteroatom-
containing alkenyl,
heteroatom-containing allcynyl, aryl, heteroaryl, alkoxy, aryloxy, amino,
silyl and combinations
thereof
[0066] As used herein the term "boryl" refers to the ¨BZ1Z2 group, where each
of and Z2 is
as defined above.
[0067] As
used herein, the term "phosphino" refers to the group ¨PZ1Z2, where each of
Z1 and Z2 is as defined above. As used herein, the term "phosphine" refers to
the group PZ1Z2Z3,
where each of Z1, Z2 and Z3 is as defined above. The term "amino" is used
herein to refer to the
group ¨NZ1Z2, where each of Z1 and Z2 is as defined above. The term "amine" is
used herein to
refer to the group NZ1Z2Z3, where each of Z1, Z2 and Z3 is as defined above.
[0068] The
term "saturated" refers to lack of double and triple bonds between atoms of
a radical group such as ethyl, cyclohexyl, pyrrolidinyl, and the like. The
term "unsaturated"
refers to the presence of one or more double and triple bonds between atoms of
a radical group
such as vinyl, acetylide, oxazolinyl, cyclohexenyl, acetyl and the like.
[0069]
Other abbreviations used herein include: "iPr" to refer to isopropyl; "tBu" to
refer to tertbutyl; "Me" to refer to methyl; "Et" to refer to ethyl; and "Ph"
refers to phenyl.
[0070] The
bridged bi-aromatic ligands disclosed herein have the following general
formula
(I):
Ri R7
R2 Ar Ar R8
R3 OH OH R9
R4 Rio
Ar Rg R12 Ar
R5 R11
(I)
wherein each of RI, R2, R3, R4, R5, R6, R7, R8, R9, RR), let and R.12
is independently selected
from the group consisting of hydride, halide, optionally substituted
hydrocarbyl, heteroatom-
containing optionally substituted hydrocarbyl, alkoxy, aryloxy, silyl, boryl,
dialkyl amino,
13
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
allcylthio, arylthio and seleno; optionally two or more R groups can combine
together into ring
structures with such ring structures having from 3 to 100 non-hydrogen atoms
in the ring; A is a
bridging group having from one to 50 non-hydrogen atoms; Y and Y' are
independently selected
from 0, S. NRa and PR' wherein Ra is optionally substituted hydrocarbyl; Ar
is, independently,
optionally substituted aryl or optionally substituted heteroaryl.
[0071] The ligands may also have the following formula (II):
Ri R7
R2 Ar Ar R8
R3 OH OH R9
R4 R10
oAo
Ar R6 R12 Ar
R5 R11
(II)
wherein each of RI, R2, R3, R4, R5, R6, R7, Rs, R9, Rif), Rt and K-12
is independently selected
from the group consisting of hydride, halide, optionally substituted
hydrocarbyl, heteroatom-
containing optionally substituted hydrocarbyl, alkoxy, aryloxy, silyl, boryl,
dialkyl amino,
alkylthio, arylthio and seleno; optionally two or more R groups can combine
together into ring
structures with such ring structures having from 3 to 100 non-hydrogen atoms
in the ring; A is a
bridging group having from one to 50 non-hydrogen atoms; Ar is, independently,
optionally
substituted aryl or optionally substituted heteroaryl.
[0072] Each of le, R2, R3, R4, R5, R6, R7, Rs, R9, Ric), and R12 may
be hydride or
optionally substituted alkyl or aryl. R2 and R8 may be optionally substituted
alkyl and each of
Ri, R3, R4, R5, R6, R7, R9, Ric), and R12 may
be hydride.
[0073] The bridging group A may be optionally substituted alkyl.
[0074] Ar may be optionally substituted phenyl, naphthyl, biphenyl,
anthracenyl,
phenanthrenyl or optionally substituted thiophene, pyridine, isoxazole,
pyrazole, pyrrole, furan,
etc. or benzo-fused analogues. The optional substituents may be alkyl groups.
[0075] The ligands may have the following structure:
14
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
R2 Ar Ar R8
OH OH
0
Ar Ar
wherein Ar may be optionally substituted phenyl, naphthyl, biphenyl,
anthracenyl,
phenanthrenyl or optionally substituted thiophene, pyridine, isoxazole,
pyrazole, pyrrole, furan,
or benzo-fused analogues and the bridging group A is a divalent alkyl.
[0076] R, and R8 may be optionally substituted alkyl.
[0077] The ligands may have the following structure:
Rx Rx
R2
40i R8
OH HO
Rx -Rx
wherein Rx, R2 and R8 are alkyl, and n = 0 to 6.
[0078] Specific ligands disclosed herein include:
cH3
H3c min 410 cH3 cH3
H cH3
H3C 3c HO
OH HO 111"
(:),.0 40
OH CH3
=
1/101 11101 H 3C C H3
H 3C
H 3C
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
H3C
OH
HO
CH3
Li gand Synthesis
[0079] The ligands disclosed herein may be prepared by a variety of
methods. In
general the ligands may be prepared by employing a tetrabromination of a
bridged phenyl
phenol and aryl coupling. The aryl coupling may be Suzuki coupling and/or
Negishi coupling.
[0080] A major advantage of the herein disclosed methods is that the
number of
reaction steps to access the ligands is low. For example, the disclosed
ligands may be prepared
from a bromophenol in three or four reaction steps.
[0081] Bridged bi-aromatic ligand syntheses disclosed in WO 03/09162
suffer from
an abundance of synthetic steps which add time and cost to a synthesis. Suzuki
couplings
disclosed in WO 03/091162 were performed on protected phenols (THP, Bn, MOM,
etc), which
add steps due to the required protections and deprotections. However, given
the protic solvents
used in these reactions, it was hypothesized that a free phenol would not
interfere with coupling.
Indeed, a Suzuki coupling with bromocresol and phenyl boronic acid was
successful and high
yielding. Other boronic acids were also coupled to bromocresol without
difficulty as shown
below:
Aryl group Yield
OH Pd(PPh3)4 OH Phenyl (1) 81%
* Br Na2003 40 Ar 2-methy 1phenyl (2) 75%
HO.,B,Ar
tol/H20
2,5-dimethylphenyl (3) 75%
OH 3,5-dimethylphenyl (4) 80%
cH, cH3
Napthyl (5) 77%
2-methy lnapthyl* (6) 49%
*pinacol borane
[0082] The following schemes illustrate general methods for the
preparation of the
ligands.
16
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[0083] Schemes 1 and 2 illustrate Suzuki coupling of a brominated phenol
with a
bridged diboronic acid.
R1 R7
R2 Re
HO OH HO OH
R1 \ B..., \ B...,
R3 OH HO Rg
R2 ill R10 Br R4 ' R4 Y R10
\A...."'Y
Pd catalyst
-3....
R3 OH R6 R12 R6 R12
R5 R11 R5 Rii
Scheme 1
R1 R7
R2 R8
R1
HO B... OH HO OH
R3 OH HO R9
R10
R2 0 Br R4 R4 R10
o.."...A/o o\A/o
Pd catalyst
_i.....
R3 OH R6 R12 R6 R12
R5 Ri 1 R5
Scheme 2
[0084] Schemes 3 and 4 illustrate tetrahalogenation of a bridged bi-
aromatic phenol.
R1 R7 R1 R7
R2 Rg R2 X X Re
R3 OH OH R9 X2
R3 OH OH R9
-1110....
R4 R10 R 4 R10
Y--Y'
R6 R12 X Rg R12 X
R5
R11 R5 R11
Scheme 3
17
CA 02982900 2017-10-13
WO 2016/172097
PCT/US2016/028268
R1 R7 Ri R7
R2 R8 R2 X X Re
R3 OH OH R9 X2 R3 OH OH R9
___________________________________ )111.1
Ret R10 R 4 O õ 10
o....s\A/ o
o R
R6 R12 X R8 R12 X
R5 R11 R5
R11
Scheme 4
100851 Schemes 5 and 6 illustrate Suzuki coupling of the tetrahalogenated
phenols with
aryl boronic acid.
Ri R7 R1 R7
R2 X X R8 R2 Ar Ar R8
R3 OH OH R9 ArB(OH)2
R3 OH OH R9
_Jo..
R4 Y-_ R 1 0 R4
\A..-----..Y. Pd catalyst Y.s(' R10
X R6
R12 X Ar Re
R12 Ar
R5
Ri 1 R5 R11
Scheme 5
R1 R7 R1 R7
R2 X X Re R2 Ar Ar Re
R3 OH OH R9 ArB (OH )2
R3 OH OH R9
-)111....
R4 0, _õ.0 Rio 4
Pd catalyst R o. R10LL
o
-----A--...--
LJL
X R6 R12 X Ar R6 R12 Ar
R5
R11 R5 R11
Scheme 6
18
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[0086] In any one of the above methods each of RI, R2, R3, R4, R5, R6, R7,
R8, R9, RH),
RH and R12 may be independently selected from the group consisting of hydride
and optionally
substituted alkyl or aryl.
[0087] In any of the above methods Y and Y' may be 0.
[0088] In any of the above methods A may be selected from the group
consisting of
optionally substituted divalent alkyl, alkenyl, alkynyl, heteroalkyl,
heteroalkenyl, heteroalkynyl,
carbocycle, heterocarbocycle, aryl, heteroaryl and silyl.
[0089] In any of the above methods A may be optionally substituted alkyl.
[0090] In any of the above methods the palladium catalyst may comprise a
palladium
phosphine compound, for example, bis(tri-tert-butylphosphine)palladium
(Pd(PPh3)4),
tetrakis(triphenylphosphine)palladium(0) (Pd(dppe)2), bis[1,2-
bis(diphenylphosphino)ethane]
palladium(0) (Pd(dppf)), 1,1'-bis(diphenylphosphino)ferrocene palladium,
(2,2'-
bis(diphenylphosphino)-1,1'-binaphthyl palladium (Pd(BINAP).
[0091] In any of the above methods the palladium catalyst may comprise a
palladium
compound and one or more phosphines. For example,
tris(dibenzylideneacetone)dipalladium(0)
(Pd2(dba)3) and Pd(OAc)2 and one or more phosphine compounds.
[0092] In any of the above methods X may be bromo or chloro. The source of
halogen
may be bromine or chlorine.
[0093] In any of the above methods the Ar-boron compound may be an
optionally
substituted aryl boronic acid or an optionally substituted heteroaryl boronic
acid.
[0094] In any of the above methods the Ar-boron compound may be an
optionally
substituted aryl boronic ester or an optionally substituted heteroaryl boronic
ester.
[0095] In any of the above methods the Ar-boron compound may be an
optionally
substituted aryl trifluoroborate or an optionally substituted heteroaryl
trifluoroborate.
[0096] In any of the above methods the Ar-boron compound may be an
optionally
substituted arylborane or an optionally substituted heteroarylborane.
[0097] In any of the above methods a base may be utilized along with the
palladium
catalyst.
[0098] In any of the above methods the base may comprise an alkali metal
carbonate,
alkali metal phosphate, alkali metal hydroxide, alkali metal alkoxide or an
amine.
[0099] The base may comprise sodium or potassium carbonate or sodium or
potassium
phosphate.
[00100] In an illustrative embodiment and referring to the structures in
Figure 1 and the
reaction scheme in Figure 3: treatment of 1,3-bis(2-bromophenoxy)propane with
n-butyllithium
19
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
followed by trimethyl borate and then HC1 afforded (Propane-1,3-
diyIbisoxy)bis(2,1-
phenylene)diboronic acid (9). The diboronic acid, 2-bromocresol, SPhos, and
potassium
phosphate were dissolved in degassed THF and water, then stirred at ambient
temperature
overnight to yield 2',21"-(Propane-1,3-diylbis(oxy))bis(5-methy141,1'-
biphenyl]-2-01) (10). The
diphenolic compound (10) was dissolved in dichloromethane and treated with
bromine. The
reaction was quenched with saturated sodium bicarbonate to yield 6',61"-
(Propane-1,3-
diylbisoxy)bis(3,3'-dibromo-5-methy141,1'-bipheny11-2-ol) (11). The brominated
compound was
combined with phenylboronic acid, SPhos and potassium phosphate in THF/H20 and
the
mixture was stirred at room temperature overnight to yield 6",6""-(Propane-1,3-
diylbisoxy)bis(5'-methy141,1':3',1":3",11"-quaterphenyl]-2'-ol) (12).
[00101] Figures 4 and 5 illustrate other exemplary reaction schemes.
Catalyst Compounds
[00102] The catalyst compounds may be prepared by any suitable synthesis
method and
the method of synthesis is not critical to the present disclosure. One useful
method of preparing
the catalyst compounds of the present disclosure is by reacting a suitable
metal compound, for
example one having a displaceable anionic ligand, with the bridged bi-aromatic
ligands of this
disclosure. Non-limiting examples of suitable metal compounds include
organometallics, metal
sulfonates, carboxylates, phosphates, organoborates (including fluoro-
containing and
other subclasses), acetonacetonates, sulfides, sulfates, tetrafluoroborates,
nitrates, perchlorates,
phenoxides, alkoxides, silicates, arsenates, borohydrides, naphthenates,
cyclooctadienes, diene
conjugated complexes, thiocynates, cyanates, and the metal cyanides. The metal
compound may
be an organometallic or metal halide. The metal compound may be an
organometallic.
[00103] The metal of the organometallic compound may be selected from
Groups 1 to 16,
or a transition metal selected from Groups 3 to 13 elements and Lanthanide
series elements. The
metal may be selected from Groups 3 to 7 elements. The metal may be a Group 4
metal,
titanium, zirconium or hafnium.
[00104] The metal compound can, for example, be a metal hydrocarbyl such
as: a metal
alkyl, a metal aryl, a metal arylallcyl; a metal silylallcyl; a metal diene, a
metal amide; or a metal
phosphide. The metal compound may be a zirconium or hafnium hydrocarbyl. The
transition
metal compound may be a zirconium arylalkyl.
[00105] An exemplary reaction is shown below:
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
HC
H3C
H3C CH3
H3C H3C CH3 H3C
CH3
0 CH3
OH OATA, 0
CI
ZrBn2Cl2 ' I CI CH3
H3C HO 0
H3C
H3C
CH3 CH3
CH3
CH3
CH3
CH3
[00106] Examples of useful and preferred metal compounds include:
(i) tetramethylzirconium, tetraethylzirconium, zirconiumdichloride (114-1,4-
dipheny1-1,3-
butadiene), bis (triethylphosphine) and zirconiumdichloride ('-14-1,4-dipheny1-
1,3-butadiene) bis
(tri-n-propylphosphine), tetrakis[trimethylsilylmethyllzirconiurn,
tetrakis[dimethylamino]zirconium, dichlorodibenzylzirconium,
chlorotribenzylzirconium,
trichlorobenzylzirconium, bis[dimethylamino]bis[benzylizirconium, and
tetrabenzylzirconium;
(ii) tetramethyltitanium, tetraethyltitanium, titaniumdichloride (i4-1,4-
dipheny1-1,3-butadiene),
bis (triethylphosphine) and titaniumdichloride (114-1,4-dipheny1-1,3-
butadiene) bis (tri-n-
propylphosphine), tetrakis[trimethylsilylmethyl]titanium,
tetrakis[dimethylamino]titanium,
dichlorodibenzyltitanium, chlorotribenzyltitanium, trichlorobenzyltitanium,
bis[dimethylamino]bis[benzyl]titanium, and tetrabenzyltitanium; and
(iii) tetramethylhafnium, tetraethylhafnium, hafniumdichloride ("ii-1,4-
dipheny1-1,3-butadiene),
bis (triethylphosphine) and hafniumdichloride (,-14-1,4-dipheny1-1,3-
butadiene) bis (tri-n-
propylphosphine), tetrakis[trimethylsilylmethyl]hafnium,
tetrakis[dimethylaminolhafnium,
dichlorodibenzylhafnium, chlorotribenzylhafnium, trichlorobenzylhafniurn,
bis[dimethylamino]bis[benzyl]hafnium, and tetrabenzylhafnium.
Catalyst and Supported Catalyst Compositions
[00107] The catalyst compositions disclosed herein may comprise one or more
catalyst
compounds as disclosed herein and one or more activators as disclosed herein.
[00108] The supported catalyst compositions as disclosed herein may
comprise one or
more supports as disclosed herein, one or more catalyst compounds as disclosed
herein and one
or more activators as disclosed herein.
[00109] The catalyst compositions and supported catalyst compositions may
comprise one
or more of the catalyst compounds as hereinbefore disclosed along with another
catalyst
compound, such as a metallocene catalyst compound or a Group V atom containing
catalyst
compound. Suitable other catalyst compounds include, but are not limited to:
21
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
(pentamethylcy cl op entadi enyl)(propylcy cl opentadi enyl)MX2,
(tetramethylcy d opentadi enyl)(propylcy opentadi enyl)MX2,
(tet ramethylcy opentadienyl)(buty icy clopentadi enyl)MX2,
Me2Si(indeny1)2MX2,
Me2Si(tetrahydroindeny1)2MX2,
(n-propyl cy cl op entadi eny1)2MX2,
(n-butyl cy cl op entadi eny1)2MX2,
(1-methyl, 3-butyl cyclopentadieny1)2MX2,
HN(CH2CH2N(2,4,6-Me3phenyI))2MX2,
HN(CH2CH2N(2,3,4,5,6-Mespheny1))2MX2,
(propyl cl op entadi enyl)(tetramethylcy cl op entadi eny OMX2,
(butyl cyclopentadieny1)2MX2,
(propyl cyclopentadieny1)2MX2, and mixtures thereof,
wherein M is Zr or Hf, and X is selected from F, Cl, Br, I, Me, benzyl,
CH2SiMe3, and
CI to C5 alkyls or alkenyls.
[00110] The
supported catalyst composition may in the form of a substantially dry powder
or be in the form of a slurry in at least one liquid vehicle. Non-limiting
examples of liquid
vehicles include mineral oils, aromatic hydrocarbons or aliphatic
hydrocarbons.
Activator Compounds
[00111] An
activator is defined in a broad sense as any combination of reagents that
increases the rate at which a transition metal compound oligomerizes or
polymerizes unsaturated
monomers, such as olefins. The catalyst compounds may be activated for
oligomerization
and/or polymerization catalysis in any manner sufficient to allow coordination
or cationic
oligomerization and/or polymerization.
[00112]
Additionally, the activator may be a Lewis-base, such as for example, diethyl
ether, dimethyl ether, ethanol, or methanol. Other activators that may be used
include those
described in WO 98/07515 such as tris nonafluorobiphenyl) fluoroaluminate.
[00113]
Combinations of activators may be used. For example, alumoxanes and ionizing
activators may be used in combinations, see for example, EP-Bl 0 573 120, WO
94/07928 and
WO 95/14044 and U.S. Pat. Nos. 5,153,157 and 5,453,410. WO 98/09996 describes
activating
metallocene catalyst compounds with perchlorates, periodates and iodates
including their
hydrates. WO 98/30602 and WO 98/30603 describe the use of lithium (2,2'-
bisphenyl-
ditrimethylsilicate).4THF as an activator for a metallocene catalyst compound.
WO 99/18135
describes the use of organo-boron-aluminum activators. EP-B1-0 781 299
describes using a
22
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
silylium salt in combination with a non-coordinating compatible anion. WO
2007/024773
suggests the use of activator-supports which may comprise a chemically-treated
solid oxide, clay
mineral, silicate mineral, or any combination thereof. Also, methods of
activation such as using
radiation (see EP-B1-0 615 981), electro-chemical oxidation, and the like are
also contemplated
as activating methods for the purposes of rendering the neutral metallocene
catalyst compound
or precursor to a metallocene cation capable of polymerizing olefins. Other
activators or
methods for activating a metallocene catalyst compound are described in, for
example, U.S. Pat.
Nos. 5,849,852, 5,859,653 and 5,869,723 and PCT WO 98/32775.
[00114] Alumoxanes may also be utilized as an activator in the catalyst
composition.
Alumoxanes are generally oligomeric compounds containing --Al(R)--0--
subunits, where R is
an alkyl group. Examples of alumoxanes include methylalumoxane (MAO), modified
methylalumoxane (MMAO), ethylalumoxane and isobutylalumoxane. Alicylalumoxanes
and
modified alkylalumoxanes are suitable as catalyst activators, particularly
when the abstractable
ligand is a halide. Mixtures of different alumoxanes and modified alumoxanes
may also be
used. For further descriptions, see U.S. Pat. Nos. 4,665,208, 4,952,540,
5,041,584, 5,091,352,
5,206,199, 5,204,419, 4,874,734, 4,924,018, 4,908,463, 4,968,827, 5,329,032,
5,248,801,
5,235,081, 5,157,137, 5,103,031 and EP 0 561 476 Al, EP 0 279 586 Bl, EP 0 516
476 A, EP 0
594 218 Al and WO 94/10180.
[00115] Alumoxanes may be produced by the hydrolysis of the respective
trialkylaluminum
compound. MMAO may be produced by the hydrolysis of trimethylaluminum and a
higher
trialkylaluminum such as triisobutylaluminum. MMAO's are generally more
soluble in
aliphatic solvents and more stable during storage. There are a variety of
methods for preparing
alumoxane and modified alumoxanes, non-limiting examples of which are
described in, for
example, U.S. Pat. Nos. 4,665,208, 4,952,540, 5,091,352, 5,206,199, 5,204,419,
4,874,734,
4,924,018, 4,908,463, 4,968,827, 5,308,815, 5,329,032, 5,248,801, 5,235,081,
5,157,137,
5,103,031, 5,391,793, 5,391,529, 5,693,838, 5,731,253, 5,731,451, 5,744,656,
5,847,177,
5,854,166, 5,856,256 and 5,939,346 and European publications EP-A-0 561 476,
EP-B1-0 279
586, EP-A-0 594-218 and EP-B1-0 586 665, WO 94/10180 and WO 99/15534. A
visually clear
methylalumoxane may be used. A cloudy or gelled alumoxane can be filtered to
produce a clear
solution or clear alumoxane can be decanted from the cloudy solution. Another
alumoxane is a
modified methyl alumoxane (MMAO) cocatalyst type 3A (commercially available
from Akzo
Chemicals, Inc. under the trade name Modified Methylalumoxane type 3A,
disclosed in U.S.
Pat. No. 5,041,584).
23
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[00116] An ionizing or stoichiometric activator, neutral or ionic, such as
tri (n-butyl)
ammonium tetrakis (pentafluorophenyl) boron, a trisperfluorophenyl boron
metalloid precursor
or a trisperfluoronapthyl boron metalloid precursor, polyhalogenated
heteroborane anions (see,
for example, WO 98/43983), boric acid (see, for example, U.S. Pat. No.
5,942,459) or
combinations thereof, may also be used. The neutral or ionic activators may be
used alone or in
combination with alumoxane or modified alumoxane activators.
[00117] Examples of neutral stoichiometric activators may include tri-
substituted boron,
tellurium, aluminum, gallium and indium or mixtures thereof. The three
substituent groups may
be each independently selected from the group of alkyls, alkenyls, halogen,
substituted alkyls,
aryls, arylhalides, alkoxy and halides. The three substituent groups may be
independently
selected from the group of halogen, mono or multicyclic (including
halosubstituted) aryls,
alkyls, and alkenyl compounds and mixtures thereof; or alkenyl groups having 1
to 20 carbon
atoms, alkyl groups having 1 to 20 carbon atoms, alkoxy groups having 1 to 20
carbon atoms
and aryl groups having 3 to 20 carbon atoms (including substituted aryls).
Alternatively, the
three groups are alkyls having 1 to 4 carbon groups, phenyl, napthyl or
mixtures thereof. The
three groups may be halogenated, for example fluorinated, aryl groups. In yet
other illustrative
examples, the neutral stoichiometric activator is trisperfluorophenyl boron or
trisperfluoronapthyl boron.
[00118] Ionic stoichiometric activator compounds may contain an active
proton, or some
other cation associated with, but not coordinated to, or only loosely
coordinated to, the
remaining ion of the ionizing compound. Such compounds and the like are
described in, for
example, European publications EP-A-0 570 982, EP-A-0 520 732, EP-A-0 495 375,
EP-B1-0
500 944, EP-A-0 277 003 and EP-A-0 277 004, and U.S. Pat. Nos. 5,153,157,
5,198,401,
5,066,741, 5,206,197, 5,241,025, 5,384,299 and 5,502,124.
Supports
[00119] The above described catalyst compounds may be combined with one or
more
supports using one of the support methods well known in the art or as
described below. For
example, the catalyst compound may be used in a supported form, such as,
deposited on,
contacted with, or incorporated within, adsorbed or absorbed in, or on the
support.
[00120] As used herein, the term "support" refers to compounds comprising
Group 2, 3, 4,
5, 13 and 14 oxides and chlorides. Suitable supports include, for example,
silica, magnesia,
titania, zirconia, montmorillonite, phyllosilicate, alumina, silica-alumina,
silica-chromium,
silica-titania, magnesium chloride, graphite, magnesia, titania, zirconia,
montmorillonite,
phyllosilicate, and the like.
24
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[00121] The
support may possess an average particle size in the range of from about 0.1 to
about 500 p.m, or from about 1 to about 200 pm, or from about 1 to about 50
pm, or from about
to about 50 pm.
[00122] The
support may have an average pore size in the range of from about 10 to about
1000 A, or about 50 to about 500 A, or 75 to about 350 A.
[00123] The
support may have a surface area in the range of from about 10 to about 700
m2/g, or from about 50 to about 500 m2/g, or from about 100 to about 400 m2/g.
[00124] The
support may have a pore volume in the range of from about 0.1 to about 4.0
cc/g, or from about 0.5 to about 3.5 cc/g, or from about 0.8 to about 3.0
cc/g.
[00125] The
support, such as an inorganic oxide, may have a surface area in the range of
from about 10 to about 700 m2/g, a pore volume in the range of from about 0.1
to about 4.0 cc/g,
and an average particle size in the range of from about 1 to about 500 gm.
Alternatively, the
support may have a surface area in the range of from about 50 to about 500
m2/g, a pore volume
of from about 0.5 to about 3.5 cc/g, and an average particle size of from
about 10 to about 200
p.m. The surface area of the support may be in the range from about 100 to
about 400 m2/g, a
pore volume of from about 0.8 to about 3.0 cc/g and an average particle size
of from about 5 to
about 100 pm.
[00126] The
catalyst compounds may be supported on the same or separate supports
together with an activator, or the activator may be used in an unsupported
form, or may be
deposited on a support different from the supported catalyst compound.
[00127]
There are various other methods in the art for supporting a polymerization
catalyst
compound. For example, the catalyst compound may contain a polymer bound
ligand as
described in, for example, U.S. Pat. Nos. 5,473,202 and 5,770,755; the
catalyst may be spray
dried as described in, for example, U.S. Pat. No. 5,648,310; the support used
with the catalyst
may be functionalized as described in European publication EP-A-0 802 203, or
at least one
substituent or leaving group is selected as described in U.S. Pat. No.
5,688,880.
Polymerization Processes
[00128]
Polymerization processes may include solution, gas phase, slurry phase and a
high pressure process or a combination thereof. In illustrative embodiments, a
gas phase or
slurry phase polymerization of one or more olefins at least one of which is
ethylene or propylene
is provided. Optionally, the reactor is a gas phase fluidized bed
polymerization reactor.
[00129] The
catalyst compositions or supported catalyst compositions as hereinbefore
described are suitable for use in any prepolymerization and/or polymerization
process over a
wide range of temperatures and pressures. The temperatures may be in the range
of from -60 C
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
to about 280 C, from 50 C to about 200 C; from 60 C to 120 C from 70 C to 100
C or from
80 C to 95 C.
[00130] The
present process may be directed toward a solution, high pressure, slurry or
gas phase polymerization process of one or more olefin monomers having from 2
to 30 carbon
atoms, preferably 2 to 12 carbon atoms, and more preferably 2 to 8 carbon
atoms. The process
is particularly well suited to the polymerization of two or more olefins or
comonomers such as
ethylene, propylene, 1-butene, 1-pentene, 4-methyl-1-pentene, 1-hexene, 1-
octene 1-decene or
the like.
[00131]
Other olefins useful in the present process include ethylenically unsaturated
monomers, diolefins having 4 to 18 carbon atoms, conjugated or nonconjugated
dienes,
polyenes, vinyl monomers and cyclic olefins. Useful monomers may include, but
are not limited
to, norbornene, norbomadiene, isobutylene, isoprene, vinylbenzocyclobutane,
styrenes, alkyl
substituted styrene, ethylidene norbomene, dicyclopentadiene and cyclopentene.
In an
illustrative embodiment of the present process, a copolymer of ethylene is
produced, where with
ethylene, a comonomer having at least one alpha-olefin having from 4 to 15
carbon atoms,
preferably from 4 to 12 carbon atoms, and most preferably from 4 to 8 carbon
atoms, is
polymerized in a gas phase process. In another embodiment of the present
process, ethylene or
propylene is polymerized with at least two different comonomers, optionally
one of which may
be a diene, to form a terpolymer.
[00132] The
present process may be directed to a polymerization process, particularly a
gas
phase or slurry phase process, for polymerizing propylene alone or with one or
more other
monomers including ethylene, and/or other olefins having from 4 to 12 carbon
atoms. The
polymerization process may comprise contacting ethylene and optionally an
alpha-olefin with
one or more of the catalyst compositions or supported catalyst compositions as
hereinbefore
described in a reactor under polymerization conditions to produce the ethylene
polymer or
cop oly mer.
[00133]
Suitable gas phase polymerization processes are described in, for example,
U.S.
Pat. Nos. 4,543,399, 4,588,790, 5,028,670, 5,317,036, 5,352,749, 5,405,922,
5,436,304,
5,453,471, 5,462,999, 5,616,661, 5,668,228, 5,627,242, 5,665,818, and
5,677,375, and European
publications EP-A-0 794 200, EP-A-0 802 202, EP-A2 0 891 990, and EP-B-634
421.
[00134] A
slurry polymerization process generally uses pressures in the range of from
about
1 to about 50 atmospheres and even greater and temperatures in the range of 0
C to about
120 C. In a shiny polymerization, a suspension of solid, particulate polymer
is formed in a
liquid polymerization diluent medium to which ethylene and comonomers and
often hydrogen
26
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
along with catalyst are added. The suspension including diluent is
intermittently or continuously
removed from the reactor where the volatile components are separated from the
polymer and
recycled, optionally after a distillation, to the reactor. The liquid diluent
employed in the
polymerization medium is typically an alkane having from 3 to 7 carbon atoms,
preferably a
branched alkane. The
medium employed should be liquid under the conditions of
polymerization and relatively inert. When a propane medium is used the process
must be
operated above the reaction diluent critical temperature and pressure.
Preferably, a hexane or an
isobutane medium is employed.
[00135] A
preferred polymerization process is referred to as a particle form
polymerization,
or a slurry process where the temperature is kept below the temperature at
which the polymer
goes into solution. Such technique is well known in the art, and described in
for instance U.S.
Pat. No. 3,248,179. Other slurry processes include those employing a loop
reactor and those
utilizing a plurality of stirred reactors in series, parallel, or combinations
thereof. Non-limiting
examples of slurry processes include continuous loop or stirred tank
processes. Also, other
examples of slurry processes are described in U.S. Pat. No. 4,613,484.
Examples of solution
processes are described in U.S. Pat. Nos. 4,271,060, 5,001,205, 5,236,998 and
5,589,555.
EXAMPLES
[00136] It
is to be understood that while the present disclosure has been described in
conjunction with the specific embodiments thereof, the foregoing description
is intended to
illustrate and not limit the scope of the disclosure. Other aspects,
advantages and modifications
will be apparent to those skilled in the art to which the disclosure pertains.
Therefore, the
following examples are put forth so as to provide those skilled in the art
with a complete
disclosure and description of how to make and use the disclosed compositions,
and are not
intended to limit the scope of the disclosure.
[00137]
General: All reagents were purchased from commercial vendors and used as
received unless otherwise noted. Analytical thin-layer chromatography (TLC)
was performed
on Selecto Plates (200 um) precoated with a fluorescent indicator.
Visualization was effected
using ultraviolet light (254 nm). Flash column chromatography was carried out
with Sigma
Aldrich Silica gel 60 A (70 ¨ 230 Mesh). NMR spectra were recorded on a Bruker
400 NMR
with chemical shifts referenced to residual solvent peaks (CDC13: 7.27 ppm for
11-1, 77.29 ppm
for I-3C; C6D6: 7.15 ppm for 11-1, 77.39 ppm for I-3C). Melting points are
reported uncorrected.
Abbreviations: SPhos - Chloro(2-dicyclohexylphosphino-2',6'-dimethoxy-1t-
bipheny1)[2-(2'-
amino-1,11-biphenyl)]palladium(II); PTSA -para-toluenesulfonic acid.
27
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[00138] General procedure for Suzuki coupling: In an appropriately sized
flask, 2-
Bromo-p-cresol (1 equiv), aryl boronic acid (1.5 equiv) and palladium
tetrakistriphenylphosphine (0.02 equiv) were dissolved in degassed toluene to
make a 0.2 M
solution with respect to the cresol. A 2 M solution of sodium carbonate (4
equiv) in degassed
H20:Me0H (4:1) was added and the mixture refluxed until completion (usually
overnight). The
reaction was cooled and the layers separated. The aqueous layer was extracted
twice with ethyl
acetate and combined organic layers were washed with brine, dried over MgSO4
or Na2SO4,
filtered, and concentrated under reduced pressure.
[00139] 5-Methy1-11,1'-bipheny11-2-ol (1): Using the above genera'
procedure, a white
solid was isolated by column chromatography in 81% yield: 11-INMR (400 MHz,
C6D6, 5): 2.10
(s, 3 H), 6.69 (m, 3 H), 7.12 (m, 2 H), 7.15 (m, 1 H), 7.35 (m, 2 H).
[00140] 2',5-Dimethy1-11,1'-biphenyl]-2-ol (2): Using the above general
procedure, a
white solid was isolated by column chromatography (30:70 actone:isohexane) in
75% yield: Rf =
0.25 (30:70 acetone:isohexane); 11-1 NMR (400 MHz, CDC13, 5): 2.20 (s, 3 H),
2.33 (s, 3 H),
4.65 (br s, 1 H), 6.70 (d, J= 6.8 Hz, 1 H), 6.94 (s, 1 H), 7.10 (m, 1 H), 7.33
(m, 4 H).
[00141] 3',5,5'-Trimethy1-11,1'-bipheny11-2-ol (3): Using the above general
procedure, a
white solid was isolated by column chromatography (30:70 actone:isohexane) in
75% yield: Rf
= 0.32 (20:80 acetone:isohexane); 1H NMR (400 MHz, CDC13, $5): 2.34 (s, 3 H),
2.41 (s, 6 H),
5.19 (br s, 1 H), 6.91 (d, J = 8.4 Hz, 1 H), 7.08 (m, 5 H).
[00142] 2',5,5'-Trimethy1-11,1'-bipheny11-2-ol (4): Using the above general
procedure, a
white solid was isolated by column chromatography (10% actone/isohexane) in
80% yield: Rf =
0.26 (10:90 acetone:isohexane);1H NMR (400 MHz, CDC13, 5): 2.15 (s, 3 H), 2.32
(s, 3 H), 2.36
(s, 3 H), 4.66 (br s, 1 H), 6.89 (d, 1= 8.4 Hz, 1 H), 6.93 (m, 1 H), 7.07 (m,
2 H), 7.21 (m, 1 H),
7.26 (m, 1 H).
[00143] 4-Methyl-2-(naphthalen-1-yl)phenol (5): Using the above general
procedure,
the product was isolated by column chromatography (30% actone:isohexane) in
77% yield as a
pale yellow oil: IFINMR (500 MHz, CDC13, 6): 2.37 (s, 3 H), 4.67 (s, 1 H),
6.98 (d, J= 9 Hz, 1
H), 7.09 (s, 1 H), 7.09 (m, 1 H), 7.56 (m, 4 H), 7.70 (d, J = 8 Hz, 1 H), 7.93
(m, 2 H); 13C NMR
(100 MHz, CDC13, 5): 20.8, 115.6, 126.0 - 134.5 (14 C), 151.2; IR (cm-1):
3519, 3045, 2920,
1590, 1496, 1333, 1276, 1183, 781.
[00144] 4-Methyl-2-(2-methylnaphthalen-1-yl)phenol (6): Following the above
general
procedure, substituting 2-methylnapthyl pinacol borane for the aryl boronic
acid, the product
was purified by silica gel chromatography (10% acetone/isohexane) in 49% yield
as a pale
yellow oil which solidified upon standing. Rf = 0.32 (30:70
acetone:isohexane); 11-1 NMR (400
28
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
MHz, CDC13, 6): 2.29 (s, 3 H), 2.36 (s, 3 H), 4.42 (s, 1 H), 6.95 (s, 1 H),
7.00 (d, J= 4.0 Hz, 1
H), 7.19 (d, J= 8 Hz, 1 H), 7.44 (m, 4 H), 7.86 (t, J= 8.0 Hz, 2 H); 13C NMR
(100 MHz, CDC13,
6): 20.7, 20.8, 115.4, 125.0, 125.5, 125.8, 126.8, 128.2, 128.7, 129.0, 130.1,
130.2, 131.5, 131.6,
132.5, 133.2, 135.8, 151.1; IR (cm'): 3498, 3426, 3050, 2922, 2860, 1617,
1594, 1497, 1335,
1275, 1228, 1188, 814.
[00145] 3-
Bromo-3',5,5'-trimethy1-11,1'-bipheny1]-2-ol (7): Phenol (3) (1 g, 4.7 mmol)
was dissolved in methylene chloride and cooled to - 35 C. Bromine (0.3 mL,
5.6 mmol) was
slowly added and the solution stirred at ambient temperature overnight. The
reaction was
quenched with saturated sodium bicarbonate solution and extracted 3 times with
methylene
chloride. The combined organic portions were washed with sodium metabisulfite
and brine,
then dried over MgSO4, filtered, and concentrated. Rf = 0.30 (10:90 ethyl
acetateisohexane); 11-1
NMR (400 MHz, CDC13, 6): 2.33 (s, 3 H), 2.49 (s, 6 H), 5.07 (br s, 1 H), 6.87
(d, J = 8.4 Hz, 1
H), 7.03 (m, 2 H), 7.19 (s, 2 H); NMR
(100 MHz, CDC13, 6): 24.7 (2 C), 25.2, 115.1, 127.4,
128.4, 129.1, 129.9, 130.3, 130.8, 131.6, 136.0, 139.4, 139.5, 150.3; IR (cm-
1): 3533, 2921,
1499, 1464, 1381, 1237, 1029, 814.
[00146]
(Butane-1,4-diyIbis(oxy))bis(2,1-phenylene)diboronic acid (8): To 1,4-bis(2-
bromophenoxy)butane (5 g, 12.5 mmol) dissolved in 40 mL THF, was added n-
butyllithium (11
mL of 2.5 M). The reddish brown solution was stirred cold for 2 h. Trimethyl
borate (5.6 mL,
50 mmol) was added as the solution slowly turned colorless and was stirred
overnight at ambient
temperature. The reaction was quenched with conc. HC1 and condensed to a white
solid. The
solid was washed with ether to give the diboronic acid in 68% yield: 11-1 NMR
(400 MHz,
DMSO-d6, 6): 1.92 (app br s, 4 H), 4.10 (app br s, 4 H), 6.93 (t, J= 8.0 Hz, 2
H), 7.00 (d, J = 8.0
Hz, 2 H), 7.36 (m, 2 H), 7.55 (m, 2 H), 7.72 (s, 4 H).
[00147] (Propane-1,3-diyIbisoxy)bis(2,1-phenylene)diboronic acid (9):
1,3-bis(2-
bromophenoxy)propane (3.1 g, 8 mmol) was prepared according to established
procedures and
dissolved in 10 mL THF, then cooled to -70 C. n-Butyllithium (6.4 mL of 2.5
M) was added
and the deep red reaction stirred for 1 h. Trimethyl borate (3.5 mL, 24 mmol)
was added as the
solution slowly turned colorless and warmed to ambient temperature over 1 h.
The reaction was
quenched with 10% HCl and extracted with three portions of ether. The combined
organic
layers were washed with brine, dried over Na2SO4, and concentrated. The
product was
recrystallized in dichloromethane, giving a white powder: I-1-1NMR (400 MHz,
DMSO, 6): 2.25
(m, 2 H), 4.19 (m, 4 H), 6.93 (m, 2 H), 6.99 (d, J = 8.0 Hz, 2 H), 7.36 (m, 2
H), 7.53 (m, 2 H),
7.74 (m, 2 H); NMR
(100 MHz, DMSO-d6, 6): 28.7, 64.1, 65.0, 111.2 (2 C), 114.5 (2 C),
120.5, 120.6, 131.5 (2 C), 138.4(2 C).
29
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
1001481 2',2"'-(Propane-1,3-diylbis(oxy))bis(5-methy1-11,1'-bipheny1]-2-01)
(10): The
above diboronic acid (600 mg, 1.89 mmol), 2-bromocresol (800 mg, 4.2 mmol),
SPhos (50 mg,
0.07 mmol), and potassium phosphate (1.6 g, 7.56 mmol) were dissolved in
degassed THF and
water, then stirred at ambient temperature overnight. The reaction mixture was
extracted with 4
portions of ether and the combined organic layers washed with brine, dried
(Na2SO4), filtered
and concentrated. Column chromatography (30% acetone/isohexane eluent) gave
the cross-
coupled product in 74% yield as a pale yellow oil: Rf = 0.37
(acetone/isohexane 30:70); 1H
NMR (400 MHz, CDC13, 6): 2.11 (qn, ./ = 6.0 Hz, 2 H), 2.33 (s, 6 H), 4.10 (t,
J= 6.0 Hz, 4 H),
6.02 (s, 2 H), 6.91 (m, 4 H), 7.05 (m, 2 H), 7.14 (m, 4 H), 7.35 (m, 4 H); 13C
NMR (100 MHz,
CDC13, 6): 20.7 (2 C), 28.9, 65.8 (2 C), 113.5 (2 C), 117.0(2 C), 122.6(2 C),
126.0(2 C), 127.7
(2 C), 129.4 (2 C), 129.9 (2 C), 130.1 (2 C), 131.8 (2 C), 132.4 (2 C), 151.5
(2 C), 154.9 (2 C);
IR (cm-1): 4307, 3028, 2923, 1597, 1499, 1445, 1270, 1229, 1113, 1054, 909,
818.
1001491 6',6"'-(Propane-1,3-diylbisoxy)bis(3,3'-dibromo-5-methy1-11,1'-
biphenyl]-2-
01) (11): The above diphenolic compound (620 mg, 1.38 mmol) was dissolved in
40 mL
dichloromethane. Bromine (0.178 mL, 3.47 mmol) was added slowly and the
reaction stirred at
room temperature for 1 h. The reaction was quenched with saturated sodium
bicarbonate, and
the organic layer washed with sodium metabisulfite and brine, then dried
(Na2SO4), filtered and
concentrated. The tetrabrominated compound was recrystallized in ether: 1H NMR
(400 IVIHz,
CDC13, 6): 2.05 (qn,J= 6.0 Hz, 2 H), 2.23 (s, 6 H), 4.00 (t, J= 6.0 Hz, 4 H),
5.72 (s, 2 H), 6.81
(d, J= 8.0 Hz, 2 H), 6.87 (d, J= 4 Hz, 2 H), 7.30 (d, J= 4.0 Hz, 2 H), 7.37
(d, J= 4.0 Hz, 2 H),
7.45 (m, 2 H); 13C NMR (100 MHz, CDC13, 6): 20.5 (2 C), 28.6, 65.2 (2 C),
111.0(2 C), 114.0
(2 C), 114.7 (2 C), 126.8 (2 C), 129.1 (2 C), 131.4 (4 C), 132.4 (2 C),
132.9(2 C), 134.4 (2 C),
147.9 (2 C), 154.6 (2 C); IR (cm-1): 3500, 2924, 1588, 1472, 1387, 1280, 1234,
1127, 1085, 808.
1001501 6",6'-(Propane-1,3-diylbisoxy)bis(5'-methyl-11,1%3',1":3",1m-
quaterpheny11-2'-ol) (12): The above brominated compound (420 mg, 0.55 mmol)
was
combined with phenylboronic acid (304 mg, 2.49 mmol), SPhos (40 mg, 0.055
mmol) and
potassium phosphate (583 mg, 2.75 mmol) in 40 mL of degassed THF/H20. The
mixture was
stirred at room temperature overnight, and then extracted with 2 portions of
ether. The
combined organic layers were washed with brine, dried (Na2SO4), filtered and
concentrated.
The residue was purified by column chromatography giving the phenylated
product as a pale
yellow solid: mp = 82 - 89 C; R = 0.44 (acetone/isohexane 30:70); 1H NMR (400
MHz,
CD2C12, 6): 2.17 (m, 2 H), 2.34 (s, 6 H), 4.18 (t, J= 8.0 Hz, 4 H), 5.8 (s, 2
H), 13C NMR (100
MHz, CD2C12, 6): 20.8 (2 C), 29.4, 65.9 (2 C), 113.7 (2 C), 127.0 - 131.7 (36
C), 135.3 (2 C),
139.3 (2 C), 140.8 (2 C), 148.7 (2 C), 155.3 (2 C).
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
[00151] Zr complex (13): The above diphenol (200 mg, 0.268 mmol) was
dissolved in
15 mL toluene and cooled to - 35 C. Dibenzylzirconium dichloride (105 mg,
0.268 mmol) was
added and the reaction heated at 60 C until a precipitate formed (approx. 3
h). Toluene was
removed and the solid washed with hexane giving the zirconium complex as a
pale cream
colored powder: 'H NMR (400 MHz, CD2C12, 6): 1.53 (m, 2 H), 2.34 (s, 3 H),
2.38 (s, 3 H), 3.76
(m, 2H), 4.15 (m, 2H), 7.14- 7.89(m, 30 H).
[00152] 2',2"'-(Butane-1,4-diylbisoxy)bis(5-methyl-I1,1'-biphenyl1-2-ol)
(14):
Diboronic acid (3 g, 9.1 mmol), 2-bromocresol (3.4 g, 18.1 mmol), SPhos (130
mg, 0.18 mmol),
and potassium phosphate (7.7 g, 36 mmol) were dissolved in 200 mL THF/H20 and
stirred at
ambient temperature overnight. The layers were separated and the aqueous layer
extracted twice
with ether. The combined organic layers were washed with 10% HC1 and brine,
then dried
(Na2SO4), filtered and concentrated. The brown residue was purified by silica
gel column
chromatography (30% ethyl acetate/isohexane), giving the product as a pale
yellow oil: II-I NMR
(400 MHz, CDC13, 6): 1.81 (m, 4 H), 2.31 (s, 6 H), 3.98 (m, 4 H), 6.22 (s, 2
H), 6.88 (m, 4 H),
7.03 (m, 6 H), 7.32 (m, 4 H); 13C NMR (100 MHz, CDC13, 6): 20.8 (2 C), 25.9 (2
C), 69.3 (2 C),
113.5 (2 C), 117.4 (2 C), 122.6 (2 C), 126.4 (2 C), 128.1 (2 C), 129.3 (2 C),
130.0 (2 C), 130.2
(2 C), 131.9 (2 C), 132.7 (2 C), 151.7 (2 C), 155.0 (2 C); IR (cm-1): 3397,
3027, 2945, 1597,
1499, 1444, 1268, 1229, 1112, 818.
[00153] 6',6"'-(Butane-1,4-diyIbis(oxy))bis(3,3'-dibromo-5-methyl-11,1'-
biphenyl1-2-
01) (15): The above diphenolic compound (700 mg, 1.5 mmol) and triethylamine
(0.30 mL, 2.2
mmol) were dissolved in 10 mL dichloromethane. Bromine (0.095 mL, 1.84 mmol)
was added
slowly and the reaction stirred at room temperature overnight. The reaction
was quenched with
saturated sodium bicarbonate, and the organic layer washed with sodium
metabisulfite and
brine, then dried (Na2SO4), filtered and concentrated. The resulting white
powder was obtained
in 95% yield by recrystallization in ether: 'H NMR (400 MHz, CDC13, 6): 1.75
(m, 4 H), 2.27 (s,
6 H), 3.93 (m, 4 H), 5.99 (s, 2 H), 6.79 (d, J= 12.0 Hz, 2 H), 6.94 (m, 2 H),
7.40 (m, 2 H), 7.45
(m, 4 H); 13C NMR (125 MHz, CDC13, 6): 20.5 (2 C), 25.8(2 C), 69.1 (2 C),
111.3 (2 C), 114.1
(2 C), 114.8 (2 C), 125.8 (2 C), 129.4 (2 C), 131.3 (2 C), 131.5 (2 C), 132.2
(2 C), 132.9 (2 C),
134.6 (2 C), 148.0 (2 C), 155.0 (2 C).
[00154] 6' ',6'
(16): The above brominated compound (700 mg, 0.908
mmol) was combined with 2,5-dimethylphenylboronic acid (613 mg, 4.1 mmol),
SPhos (64 mg,
0.09 mmol) and potassium phosphate (1.5 g, 7.2 mmol) in 80 mL of degassed
THF/H20. The
mixture was stirred at room temperature overnight, and then extracted with 2
portions of ether.
31
CA 02982900 2017-10-13
WO 2016/172097 PCT/US2016/028268
The combined organic layers were washed with brine, dried (Na2SO4), filtered
and concentrated.
The residue was purified by column chromatography giving the phenylated
product as a pale
yellow solid: 11-1 NMR (400 MHz, CDC13, 6): 1.83 (m, 4 H), 2.16 - 2.36 (m, 30
H), 4.03 (4 H),
5.96 (s, 2 H), 6.95 (m, 4 H), 7.07 (m, 10 H), 7.28 (m, 2 H), 7.36 (m, 2 H);
13C NMR (100 MHz,
CDC13, 6): 20.4 - 23.5 (10 C), 26.7(2 C), 69.8(2 C), 113.5(2 C), 118.2(2 C),
126.9- 141.8 (42
C), 149.2 (2 C), 154.9 (2 C); IR (cm'): 3539, 3384, 3015, 2920, 1609, 1493,
1461, 1382, 1228,
908, 811.
[00155] Zr complex (17): To compound (16) (200 mg, 0.23 mmol) dissolved in
15 mL
toluene, was added dibenzylzirconium dichloride (90 mg, 0.23 mmol) at - 35 C.
The mixture
was heated at 70 C for 3 h, then concentrated to an oil. Upon dissolving the
oil in hexane, a
while solid precipitated and was washed with pentane: IFINMR (400 MHz, CD2C12,
6): 1.41 (m,
4 H), 2.26 -2.47 (m, 30 H), 4.13 (m, 2 H), 4.54 (m, 2 H), 5.95 (d, J = 8.0 Hz,
2 H), 7.30 (m, 22
H).
[00156] 6',6"'-(Butane-1,4-diylbisoxy)bis(5-methyl-3,3'-di(naphthalen-l-y1)-
11,1'-
bipheny1]-2-01) (18): Brominated compound (15) (400 mg, 0.519 mmol) was
combined with 2-
naphthylboronic acid (402 mg, 2.3 mmol), palladium tetrakistriphenylphosphine
(30 mg, 0.025
mmol) and sodium carbonate (770 mg, 7.26 mmol) in 20 mL of degassed tol/H20.
The mixture
was heated at 90 C overnight, and then extracted with 2 portions of ether.
The combined
organic layers were washed with brine, dried (Na2SO4), filtered and
concentrated. The residue
was purified by column chromatography (20% acetone/isohexane) giving the
tetranaphthylated
product as a white powder in 52% yield: 1-11 NMR (400 MHz, CDC13, 6): 1.9 (m,
4 H), 2.31 (s, 6
H), 4.04 (m, 4 H), 7.00 (m, 2 H), 7.10 (m, 2 H), 7.21 (m, 2 H), 7.50 (m, 16
H), 7.62 (in. 2 H),
7.91 (m, 10 H), 8.03 (d, J = 12.4 Hz, 2 H); 13C NMR (100 MHz, CDC13, 6): 20.7
(2 C), 23.1,
26.2, 69.3 (2 C), 112.9(2 C), 125.6 - 134.4 (52 C), 134.8 (2 C), 137.1 (2 C),
139.8 (2 C), 149.3
(2 C), 154.7 (2 C); IR (cm-1): 3393, 3043, 2950, 1710, 1600, 1497, 1465, 1226,
779.
[00157] Zr complex (19): Ligand (18) (200 mg, 0.208 mmol) was dissolved in
15 mL
toluene and cooled to - 35 C. Dibenzylzirconium dichloride (82 mg, 0.208
mmol) was added
and the reaction heated at 90 C until a precipitate formed (approx. 3 h).
Toluene was removed
and the solid washed with hexane giving the zirconium complex as a white
powder: NMR
(500 MHz, tol-d8, 90 C, 6): 1.64 (m, 4 H), 2.12 (s, 3 H), 2.17 (s, 3 H), 3.72
(m, 4 H), 5.51 (s, 1
H), 6.78 (d, J= 8.5 Hz, 2 H), 7.06 (m, 2 H), 7.30 (m, 19 H), 7.64 (m, 10 H),
7.87 (d, J= 8.5 Hz,
2 H), 8.06 (d, J = 8.5 Hz, 2 H).
[00158] General procedure for supporting catalysts: The zirconium complex,
typically
between 15 to 30 mg, was dissolved in toluene and a solution of
methylalumoxane (MAO;
32
84112363
Albemarle, 30 wt. % in toluene) added. Silica gel (Grace-Davison 757
pretreated at 600 C) was
added and the slurry stirred until completely mixed (approximately 5 minutes).
Toluene was
then removed under vacuum to give a dry free flowing powder.
Laboratory Polymerization Tests
[00159] A 2L autoclave was charged with fine granular sodium chloride
under an inert N2
atmosphere. 5g of methylalumoxane treated silica was added to the reactor by
pressuring it in
with a N2 push. The reactor temperature was set to 85 C. The reactor was
composed with
hydrogen, 1-hexene and ethylene such that the set-point reactor pressure was
220 psig and the 1-
hexene/ethylene mole ratio set. A pre-weighed charge of catalyst, between 10-
15 mg, was
pressured into the reactor. The pressure set-point of the reactor was set to
220 psig. Ethylene
was fed to the reactor to maintain this set-point. H2 and 1-hexene were also
fed to the reactor
such that their set-point concentration and C6/C2 ratio, respectively, were
maintained. After one
hour of run time, the polymer product was recovered and weighed.
[00160] The below Table collects the results of polymerization tests and
polymer
characterization:
Zirconium C6/C2 Productivity Recovery
Mn Mw Mz Mw/Mn Me
Compound Ratio 12/2 cat] 0/0
(13) 0.1000 2061 214020
642307 1414075 3.0 81.4 21.5
(17) 0.0800 3340 231817
776061 2055128 3.35 21.1 15.1
(17) 0.0150 1665 648027
1279746 2135760 1.97 100.8 2.2
(19) 0.1200 5182 292927
1039138 2703966 3.55 14 21.1_
(19) 0.0200 3302 799696
1524295 2427565 1.91 49.6 4.1
[00161] All of the catalysts showed good productivity and made high
molecular weight
ethylene-l-hexene copolymers. 'Recovery %' refers to the % polymer recovered
from the
reactor. 'Me' refers to the number of short chain branch end groups as
measured by NMR
spectroscopy.
[00162] For the sake of brevity, only certain ranges are explicitly
disclosed herein.
However, ranges from any lower limit may be combined with any upper limit to
recite a range
not explicitly recited, as well as, ranges from any lower limit may be
combined with any other
lower limit to recite a range not explicitly recited, in the same way, ranges
from any upper limit
may be combined with any other upper limit to recite a range not explicitly
recited.
33
Date Regue/Date Received 2022-10-17