Note: Descriptions are shown in the official language in which they were submitted.
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-1 -
METHOD FOR PRODUCING GALACTOOLIGOSACCHARIDES FROM
LACTOSE
BACKGROUND
1. Field
This disclosure relates to the enzymatic preparation of galactooligosaccharide
(GOS) from lactose. More
particularly, this disclosure relates to the
sequential use of two different microbial lactase enzymes to maximize the
degree of transgalactosylation during the digestion of lactose.
2. Description of Related Art
Galactooligosaccharides (GOS) are non-digestible carbohydrates that serve
as the building block of oligosaccharides in human milk. GOS modulate the
growth and activity of gastrointestinal microorganisms, and are therefore
believed to promote a healthy balance of microorganisms in the gut. Among
other things, GOS are believed to reduce levels of blood serum cholesterol,
improve mineral absorption, and prevent colon cancer development. The
properties of GOS depend significantly on the chemical composition,
structure, and degree of polymerization (DP).
GOS can be formed by the digestion of lactose with 13-D-galactoside
galactohydrolases. p-D-galactoside galactohydrolases catalyze the hydrolysis
of the galactosyl moiety from the non-reducing end of lactose. In addition, p-
D-
galactoside galactohydrolases can catalyze transgalactosylation in which a
galactosyl moiety is transferred to a nucleophilic acceptor other than water,
i.e. potentially any sugar present in a reaction medium. Transgalactosylation
is a kinetically controlled reaction, and represents competition between the
reactions of hydrolysis and synthesis. The ability to favor synthesis over
hydrolysis depends on several factors, including the origin of the 13-D-
galactoside galactohydrolase and the initial composition of acceptor sugars in
the medium (e.g. lactose and galactose) with which the enzymes are
presented. If lactose is the initial substrate, transgalactosylation results
in the
production of GOS comprising a mixture of di- (DP2), tri- (DP3), and even
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-2-
higher oligosaccharides (DP4+) with or without a terminal glucose. The
chemical structure and composition of a GOS (e.g. the number of hexose
moieties and the types of linkages) affects its properties, such as the
fermentation pattern by probiotic bacteria in the gut. The chemical
compositions, structure, degree of polymerization, and yield of GOS also
depends on the origin of the p-D-galactoside galactohydrolases utilized.
Many adults are lactose intolerant, and thus it is desirable to hydrolyze as
much lactose as possible during the preparation of GOS from lactose.
However, reaction conditions that favor the enzymatic digestion of lactose to,
for example, less than 20% of the initial lactose concentration tend to also
favor the digestion of GOS that is synthesized. Accordingly, reducing lactose
concentration may result in reduced yield of GOS.
SUMMARY
This disclosure relates to a method of producing galactooligosaccharide
(GOS) from lactose. The method includes incubating an initial aqueous
solution comprising lactose at an initial concentration with an acid fungal
lactase to produce an intermediate aqueous solution comprising lactose and
GOS in which the concentration of lactose is about 30% to about 70% of the
initial concentration of the initial aqueous solution; adding a yeast lactase
to
the intermediate aqueous solution; and incubating the intermediate aqueous
solution comprising the yeast lactase to produce a final aqueous solution in
which the concentration of lactose is between 0% and 20% of the initial
concentration of the initial aqueous solution. Incubating the initial aqueous
solution to produce the intermediate aqueous may involve incubating the
initial aqueous solution to produce the intermediate aqueous having about
40% of the initial concentration of lactose the initial aqueous solution.
Incubating the initial aqueous solution to produce the intermediate aqueous
may involve incubating the initial aqueous solution to produce the
intermediate aqueous comprising 49% to 52% DP2 sugar (w/w) of total sugar
in the intermediate aqueous solution. Incubating the intermediate aqueous
solution with the yeast lactase to produce the final aqueous solution, may
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-3-
involve incubating the intermediate aqueous solution to produce the final
aqueous solution comprising 23.5 % to 25% DP2 sugar (w/w) of total sugar in
the final aqueous solution.
The method may further include adjusting the pH of the intermediate aqueous
solution to between 5.5 and 9.0 with KOH, MgCl2, and citric acid prior to
adding the yeast lactase. Adjusting the pH of the intermediate aqueous
solution to between 5.5 and 9.0 with KOH, MgCl2, and citric acid may include
adjusting the pH to between 6.0 and 7.5. Adjusting the pH of the intermediate
aqueous solution to between 5.5 and 9.0 with KOH, MgCl2, and citric acid may
include adjusting the pH to about 6.8. Adjusting the pH of the intermediate
aqueous solution with KOH, MgCl2, and citric acid may include sequentially
adding KOH, MgCl2, and citric acid to the intermediate aqueous solution.
Sequentially adding KOH, MgCl2, and citric acid to the intermediate aqueous
solution comprises, in sequential order: adjusting the pH of the intermediate
aqueous solution to about 9.2 with KOH; adding about 0.16 g of MgCl2 per
100 g of aqueous solution to the intermediate aqueous solution; and adjusting
the pH of the intermediate aqueous solution from about 9.1 to about 6.8.
The acid fungal lactase may be a fungal 8-D-galactoside galactohydrolase.
The fungal 13-D-galactoside galactohydrolase may be derived from an
Aspergillus species. The Aspergillus species may be Aspergillus oryzae. The
concentration of the acid fungal lactase may be expressed in terms of lactase
units (LU) per gram of lactose in the solution. The concentration of the acid
fungal lactase in the initial aqueous solution may be between 1 and 300 LU
per gram of lactose in the initial aqueous solution. The
concentration of
the acid fungal lactase may be between about 10 and about 20 LU per gram
of lactose in the initial aqueous solution. The concentration of the acid
fungal
lactase may be between about 15 and about 17 LU per gram of lactose in the
initial aqueous solution. The concentration of the acid fungal lactase may be
about 16.7 LU per gram of lactose in the initial aqueous solution.
Alternatively, the concentration of the acid fungal lactase may be about 5.6
LU
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-4-
per gram of lactose in the initial aqueous solution, or about 5.8 LU per gram
of
lactose in the initial aqueous solution.
The yeast neutral lactase may be a yeast p-D-galactoside galactohydrolase.
The yeast 13-D-galactoside galactohydrolase may be derived from a
Kluyveromyces species. The Kluyveromyces species may be Kluyveromyces
lactis.
Adding the yeast neutral lactase to the intermediate aqueous solution may
include adding the yeast lactase to a concentration of between 1 and 50 LU
per gram of lactose in the intermediate aqueous solution. Adding the yeast
neutral lactase to the intermediate aqueous solution may include adding the
yeast lactase to a concentration of about 4 to about 5 LU per gram of lactose
in the intermediate aqueous solution. Adding the yeast neutral lactase to the
intermediate aqueous solution may include adding the yeast lactase to a
concentration of about 4.7 LU per gram of lactose in the intermediate aqueous
solution. Adding the yeast neutral lactase to the intermediate aqueous
solution may include adding the yeast lactase to a concentration of about 4.4
LU per gram of lactose in the intermediate aqueous solution.
The initial concentration of lactose in the initial aqueous solution may be
between 15 and 63 Bx. The initial concentration of lactose in the initial
aqueous solution may be between about 30 Bx and about 60 Bx. The initial
concentration of lactose in the initial aqueous solution may be about 45 Bx.
The initial concentration of lactose in the initial aqueous solution may be
about
53 Bx.
The initial aqueous solution may be incubated with the fungal acid lactase at
a
temperature between about 25 and 65 C. The temperature may be between
about 40 and about 55 C. The initial aqueous solution may be incubated with
the fungal lactase at a temperature of about 53.5 C.
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-5-
The initial aqueous solution may be incubated with the fungal lactase at a pH
between about 2.5 and about 8Ø The initial aqueous solution may be
incubated with the fungal lactase at a pH between about 3.5 and about 6.5. In
particular embodiments, the initial aqueous solution is incubated with the
fungal lactase at a pH between about 4.5 and about 5.5.
In some embodiments, the method includes deactivating the fungal acid
lactase prior to adding the yeast neutral lactase. In some embodiments,
deactivating the fungal lactase comprises adjusting the pH of the intermediate
aqueous solution to about 2 or less. In some embodiments, deactivating the
fungal acid lactase includes adjusting the pH of the intermediate aqueous
solution to about 2. The pH of the intermediate aqueous solution may be
adjusted with hydrochloric acid (HCI) to deactivate the fungal lactase. In
some
embodiments, deactivating the fungal acid lactase includes heating to above
72 C.
The intermediate aqueous solution may incubated with the yeast neutral
lactase at a temperature between about 4 and about 50 C. In some
embodiments, the intermediate aqueous solution is incubated with the yeast
lactase at a temperature between about 30 and about 45 C. In some
embodiments, the intermediate aqueous solution is incubated with the yeast
lactase at a temperature of about 36.5 C.
The method may further include deactivating the yeast lactase. In some
embodiments, deactivating the yeast lactase includes adjusting the pH of the
final aqueous solution to about pH 5.5. In some embodiments, the pH of the
final aqueous solution is adjusted to about pH 5.5 with citric acid. In some
embodiments, deactivating the yeast lactase includes incubating the final
aqueous solution at 72 C.
The method may further include partially removing glucose and galactose
from the final aqueous solution by chromatography to produce a GOS-
enriched solution.
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-6-
The method may further include removing the fungal acid lactase, the yeast
neutral lactase, glucose and galactose from the final aqueous solution by
chromatography.
In some embodiments, the fungal acid lactase and the yeast neutral lactase,
is removed from the final aqueous solution by ion exchange chromatography.
In some embodiments, the glucose and/or galactose is at least partially
removed from the final aqueous solution by ion exchange, filtration,
chromatographic separation, or additional fermentation reactions. In some
embodiments, chromatographic separation comprises simulated moving bed
chromatography.
This disclosure further relates to a galactooligosaccharide (GOS) syrup
produced according to a method as described above. In some embodiments,
the GOS syrup is at least 40% GOS w/w of the total carbohydrate in the GOS
syrup. In some embodiments, the GOS syrup is at least 65% GOS w/w of the
total carbohydrate in the GOS syrup.
In some embodiments, the wherein ratio of DP2:DP3:DP4 in the GOS syrup is
about 2:3:1.
This disclosure also relates generally to the use of a p-D-galactoside
galactohydrolase derived from Aspergillus oryzae in combination with a p-D-
galactoside galactohydrolase derived from Kluyveromyces lactis in the
preparation of galactooligosaccharide (GOS) syrup from an aqueous solution
comprising lactose, wherein the GOS syrup is at least about 40% GOS w/w of
the total carbohydrate in the GOS syrup. The p-D-galactoside
galactohydrolase derived from Aspergillus oryzae is for incubation with the
aqueous solution prior to incubation of the aqueous solution with the p-D-
galactoside galactohydrolase derived from Kluyveromyces lactis.
CA 2992497
- 7 -
In some embodiments, the GOS syrup may be at least about 60% GOS w/w of the
total
carbohydrate in the GOS syrup. In some embodiments, the GOS syrup may be about
65%
GOS w/w of the total carbohydrate in the GOS syrup.
This disclosure also relates generally to the use of a f3-D-galactoside
galactohydrolase derived
from Kluyveromyces lactis for increasing the amount of galactooligosaccharide
(GOS) in an
aqueous solution comprising lactose that has been previously treated with a p-
D-galactoside
galactohydrolase derived from Aspergillus oryzae. In some embodiments, the
amount of GOS
may be increased to at least 40% w/w of total carbohydrates in the solution.
In some
embodiments, the diversity of GOS may be increased.
This disclosure also relates generally to the use of a p-D-galactoside
galactohydrolase derived
from Aspergillus oryzae in combination with a p-D-galactoside galactohydrolase
derived from
Kluyveromyces lactis in reducing the concentration of lactose in an aqueous
solution to less
than 20% w/w of the initial concentration of lactose. The P-D-galactoside
galactohydrolase
derived from Aspergillus oryzae is for incubation with the aqueous solution
prior to incubation
of the aqueous solution with the p-D-galactoside galactohydrolase derived from
Kluyveromyces lactis.
Various embodiments of the claimed invention relate to a method of producing
galactooligosaccharide (GOS) from lactose, the method comprising: incubating
an initial
aqueous solution comprising lactose at an initial concentration with an acid
lactase, wherein
the acid lactase is an acid P-D-galactoside galactohydrolase derived from
Aspergillus otyzae,
to produce an intermediate aqueous solution comprising lactose and GOS in
which the
concentration of lactose is about 30% to about 70% of the initial
concentration of the initial
aqueous solution, and in which DP2 sugar is about 49% to about 52% by weight
of total sugar
in the intermediate aqueous solution; adjusting the intermediate aqueous
solution, wherein
adjusting the intermediate aqueous solution comprises adjusting the pH of the
intermediate
aqueous solution to between 5.5 and 9.0; adding a neutral lactase to the
intermediate aqueous
solution, wherein the neutral lactase is a neutral p-D-galactoside
galactohydrolase derived
from a Kluyveromyces lactis; and incubating the intermediate aqueous solution
comprising the
CA 2992497 2020-01-15
CA 2992497
- 7a -
neutral lactase to produce a final aqueous solution in which the concentration
of lactose is
about 20% or less of the initial concentration of the initial aqueous
solution. Various
embodiments of the claimed invention relate to use of an acid lactase in
combination with a
neutral lactase in the preparation of galactooligosaccharide (GOS) syrup from
an aqueous
mixture comprising lactose, wherein the GOS syrup comprises at least about 40%
GOS w/w of
the total carbohydrate in the GOS syrup, and wherein the GOS syrup comprises
about 23.5%
to about 25% DP2 sugar by weight of total sugar in the aqueous solution.
Various embodiments of the claimed invention relate to a method of minimizing
the turbidity of
a reaction mixture comprising a solution comprising lactose and
galactoligosaccharides as it is
adjusted from a pH of about 4.5 to 5.5 for incubation with a neutral lactase
at a pH of about 6.0
to about 7.5, the method comprising, in sequential order: adjusting the pH of
the solution to
about 9.2 with KOH; adding about 0.16 g of MgCl2 per 100 g of the solution to
the intermediate
aqueous solution; and adjusting the pH of the solution with an acid to between
about 6.0 and
about 7.5.
Various embodiments of the claimed invention relate to a method of minimizing
the turbidity of
a reaction mixture comprising a solution comprising lactose and
galactoligosaccharides as it is
adjusted from basic pH toward a neutral pH for incubation with a neutral
lactase, the method
comprising adjusting a salt concentration of the solution with MgCl2 before
adding an acid to
reduce the pH toward the neutral pH.
Various embodiments of the claimed invention relate to a method of producing
galactooligosaccharide (GOS) from lactose, the method comprising: incubating
an initial
aqueous solution comprising lactose at an initial concentration with an acid
13-D-galactoside
galactohydrolase derived from Aspergillus oryzae to produce an intermediate
aqueous solution
comprising lactose and GOS in which the concentration of lactose is about 30%
to about 70%
of the initial concentration of the initial aqueous solution; adjusting the
intermediate aqueous
solution, wherein adjusting the intermediate aqueous solution comprises
adjusting the pH of
the intermediate aqueous solution to between 5.5 and 9.0; adding neutral p-D-
galactoside
galactohydrolase derived from Kiuyveromyces lactis to the intermediate aqueous
solution; and
incubating the intermediate aqueous solution comprising the neutral 13-D-
galactoside
CA 2992497 2020-01-15
CA 2992497
- 7b -
galactohydrolase until DP2 sugar is about 23.5% to 25% of total sugar by
weight to produce a
final aqueous solution in which the concentration of lactose is about 20% or
less of the initial
concentration of the initial aqueous solution.
Various embodiments of the claimed invention relate to use of an acid P-D-
galactoside
galactohydrolase derived from Aspergillus otyzae in combination with a neutral
13-D-
galactoside galactohydrolase derived from Kluyveromyces lactis in the
preparation of
galactooligosaccharide (GOS) syrup from an aqueous mixture comprising lactose,
wherein the
GOS syrup comprises at least about 40% GOS w/w of the total carbohydrate in
the GOS
syrup.
Other aspects and features of the present invention will become apparent to
those ordinarily
skilled in the art upon review of the following description of specific
embodiments of the invention
in conjunction with the accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
In drawings which illustrate embodiments of the invention,
Figure 1 is a flow diagram of a method of producing GOS syrup as
disclosed herein in
Example 5.
CA 2992497 2020-01-15
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-8-
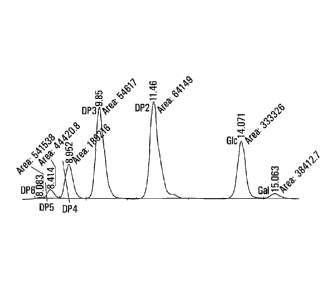
Figure 2 is a HPLC chromatogram following primary
transgalactosylation of
lactose using fungal p-D-galactoside galactohydrolases from
Aspergillus oryzae as disclosed herein in Example 5.
Figure 3 is a HPLC chromatogram following
secondary
transgalactosylation of lactose using yeast p-D-galactoside
galactohydrolases from Kluyveromyces as disclosed herein in
Example 5.
Figure 4 is a flow diagram of a method of producing GOS syrup as
disclosed herein in Example 6.
Figure 5 is a HPLC chromatogram following primary
transgalactosylation of
lactose using fungal p-D-galactoside galactohydrolases from
Aspergillus oiyzae as disclosed herein in Example 6.
Figure 6 is a HPLC chromatogram following secondary
transgalactosylation
of lactose using yeast p-D-galactoside galactohydrolases from
Kluyveromyces as disclosed herein in Example 6.
Figure 7 is a HPLC chromatogram of final product after purification
and
enrichment as disclosed herein in Example 6.
DETAILED DESCRIPTION
Definitions
"DP" as used herein refers to the degree of polymerization of the GOS. A
disaccharide GOS is characterized as a "DP2". A trisaccharide GOS is
characterized as a "DP3". A tetrasaccharide GOS is characterized as a "DP4".
The skilled person will understand that each grouping may include a plurality
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-9-
of species of GOS which differ in terms of the sequence of sugar moieties and
the linkages between moieties.
"Initial aqueous solution" as used herein refers to the lactose solution that
is
prepared for and is digested by the acid fungal lactase as the primarily
active
lactase.
"Initial concentration of lactose" as used herein refers to the amount of
lactose
that is added to create the initial aqueous solution, including any lactose
that
may be added to the initial aqueous solution after incubation with the acid
fungal lactase has commenced.
"Intermediate aqueous solution" as used herein refers to the resulting lactose
solution upon the effective termination of the digestion of the initial
aqueous
solution by the acid fungal lactase that is then digested by the yeast neutral
lactase.
"Final aqueous solution" as used herein refers to the resulting lactose
solution
upon the effective termination of the digestion of the intermediate aqueous
solution by the yeast neutral lactase.
This disclosure relates to methods of producing galactooligosaccharide (GOS)
from lactose using a combination of acidic lactases and neutral lactases. More
particularly, the method comprises incubating an aqueous solution comprising
lactose with an acid fungal lactase. The acidic fungal lactase hydrolyses the
lactose in the solution to galactose and glucose. The lactase further
catalyzes
transgalactosylation reactions in which the galactosyl moiety is transferred
to
potentially any sugar moiety present in the solution (e.g. galactose, glucose,
lactose, etc.) to produce GOS comprising a mixture of DP2, DP3, DP4, DP5,
and even higher order oligosaccharides.
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-10-
Primary Digestion with an Acid Fungal Lactase
In various embodiments of the methods disclosed herein, the acid fungal
lactase is a fungal p-c-galactoside galactohydrolase. The p-c-galactoside
galactohydrolase may be derived an Aspergillus species. In particular
embodiments, the p-c-galactoside galactohydrolase is derived from
Aspergillus oryzae, such as the p-c-galactoside galactohydrolase available
from Enzyme Development Corporation (New York) as ENZECOTM Fungal
Lactase Concentrate. The skilled person will understand that the
determination of lactase units (LU) will be specified on the TDS for an
enzyme. One LU may be defined as that quantity of enzyme which will
liberate 1.0 pmol/min of o-nitrophenol under the conditions of the assay
specified in the TDS. The concentration of the acid fungal lactase in the
initial
aqueous solution may be between 1 and 300 LU per gram of lactose in the
initial aqueous solution. The concentration of the acid fungal lactase may be
between about 10 and about 20 LU per gram of lactose in the initial aqueous
solution. The concentration of the acid fungal lactase may be between about
15 and about 17 LU per gram of lactose in the initial aqueous solution. In
particular embodiments, the concentration of the acid fungal lactase may be
about 16.7 LU per gram of lactose in the initial aqueous solution. In
particular
embodiments, the concentration of the acid fungal lactase may be about 5.6
LU per gram of lactose in the initial aqueous solution. In
particular
embodiments, the concentration of the acid fungal lactase may be about 5.8
LU per gram of lactose in the initial aqueous solution. Nevertheless, the
skilled person will understand that the methods disclosed herein may be
performed with a wide range of acid lactase concentrations depending on a
number of factors including the initial concentration of lactose in the
aqueous
solution, the length of time for which the reaction is allowed to proceed, the
pH, and the reaction temperature.
The source of lactose may vary. The lactose can be provided in the form of
milk permeate. Alternatively, the lactose can be provided as edible
crystalline
lactose commonly available from commercial suppliers. The initial
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-11-
concentration of lactose in the initial aqueous solution should be in the
range
of 15 to 63 Bx. Nevertheless, the skilled person will understand that, for
commercial purposes, the initial concentration of lactose should be higher
than 15 Bx, as lower concentration of lactose favors hydrolysis over the
transgalactosylation, thereby leading to lower GOS yields. Moreover, lower
initial concentrations of lactose necessitate larger volumes to be processed
in
order to obtain the same amount of products, and thus more resources for
downstream separation such as chromatographic apparatuses and
evaporators. Accordingly, the initial concentration of lactose and in an
initial
aqueous solution will preferably be between about 30 Bx and about 60 Bx. In
particular embodiments, the initial concentration of lactose and in the
initial
aqueous solution is about 45 Bx. In particular embodiments, the initial
concentration of lactose and in the initial aqueous solution is about 53 Bx.
The pH of the initial aqueous solution should be in the range of about 2.5 to
about 8Ø The skilled person will understand, however, that the pH of the
initial aqueous solution should be close to the optimal pH for the enzyme.
Accordingly, in some embodiments, the pH of the initial aqueous solution will
be between about 3.5 and about 6.5. In some embodiments, the pH of the
initial aqueous solution will be between about 4.5 and about 5.5. For example,
ENZECOTM Fungal Lactase Concentrate has activity within a pH range of
about 2.5 to about 2.8, although the activity may be slow outside a pH range
of about 3.5 to about 6.5. The ENZECOTM Fungal Lactase Concentrate, for
example, has a pH optimum of between 4.5 and 5Ø
The skilled person will understand that the pH of a solution comprising
lactose
may vary depending on the concentration of lactose and the source of
lactose. Accordingly, it may be necessary to adjust the pH of the initial
aqueous solution within the suitable pH range to bring the pH of the initial
aqueous solution within the desired range.
The initial aqueous solution is incubated with the fungal lactase at a
temperature between about 35 and about 65 C. ENZECOTM Fungal Lactase
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-12-
Concentrate, for example, has a temperature optimum of 55 C at pH 4.5 and
6.5. Thus, in some embodiments, the initial aqueous solution is incubated with
the acid fungal lactase at a temperature between about 50 and about 56.5 C.
In some embodiments, the initial aqueous solution is incubated with the acid
fungal lactase at a temperature between about 50 and about 55 C. In
particular embodiments, the initial aqueous solution is incubated with the
acid
fungal lactase at a temperature of about 53.5 C.
The skilled person will understand that the methods disclosed herein are not
limited by any specific reaction time for the incubation of the initial
aqueous
solution with the acid fungal lactase. Rather, the reaction is allowed to
proceed until about 20% to about 70% of the lactose provided in the initial
aqueous solution is hydrolyzed (i.e. until the concentration of lactose is
between about 20% to about 70% of the initial concentration of lactose in the
initial aqueous solution). In particular embodiments, the reaction is allowed
to
proceed until about 40% of the lactose provided in the initial aqueous
solution
is hydrolyzed (i.e. until the concentration of lactose is about 40% of the
initial
concentration of lactose in the initial aqueous solution) and/or until DP2
sugars comprise 49% to 52% (w/w) of total sugar in the intermediate aqueous
solution. Accordingly, the concentration of lactose and other sugars in the
initial aqueous solution may be monitored from time to time in order to
identify
an appropriate time to end the incubation with the acid fungal lactase. The
skilled person will understand that incubation time depends on a combination
of temperature, initial lactose concentration, pH, and lactase concentration.
Reactions may be run quickly with a large concentration of enzyme if enzyme
cost in not important. Alternatively, enzyme costs may be saved if a reaction
is carried out more slowly. Parameters may also be adjusted depending on
how the reaction time is to be logistically tied in downstream processes.
Secondary Digestion with a Neutral Yeast Lactase
Once the desired concentration of lactose in the aqueous solution (and/or a
DP2 sugar concentration of about 49% to 52% (w/w) of total sugar in the
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-13-
intermediate aqueous solution) is achieved, this intermediate aqueous
solution is incubated with a yeast neutral lactase. Prior to adding the yeast
neutral lactase, it may be preferable to deactivate the acid fungal lactase.
Deactivating the acid fungal lactase may involve adjusting the pH of the
intermediate aqueous solution to about 2 or less with, for example, HCI.
Deactivating the acid fungal lactase seeks to minimize hydrolysis of GOS by
the acid fungal lactase, and thereby maximize GOS yield. However, the
skilled person will understand that active steps to deactivate of the acid
fungal
lactase may not be completely necessary.
The neutral yeast lactase is added to the intermediate aqueous solution
comprising GOS and about 20 to about 70% of the initial lactose to a
concentration. In various embodiments of the methods disclosed herein, the
neutral yeast lactase is a yeast p-D-galactoside galactohydrolase. The pi-D-
galactoside galactohydrolase may be derived from a Kluyveromyces species.
In particular embodiments, the p-D-galactoside galactohydrolase is derived
from Kluyveromyces lactis, such as the p-D-galactoside galactohydrolase
available from Enzyme Development Corporation (New York) as ENZECOTM
Lactase NL 2.5X. The yeast neutral lactase may be added to the intermediate
aqueous solution at a concentration of between 1 and 50 LU per gram of
lactose in the intermediate aqueous solution. The yeast neutral lactase may
be added to the intermediate aqueous solution at a concentration of about 4
to about 5 LU/g lactose in the intermediate aqueous solution. The yeast
neutral lactase may be added to the intermediate aqueous solution at a
concentration of about 4.7 LU per gram of lactose in the intermediate aqueous
solution. The yeast neutral lactase may be added to the intermediate aqueous
solution at a concentration of about 4.4 LU per gram of lactose in the
intermediate aqueous solution.
Nevertheless, the skilled person will
understand that the methods disclosed herein may be performed with a wide
range of yeast lactase concentrations depending on a number of factors
including the initial concentration of lactose in the intermediate aqueous
solution, the length of time for which the reaction is allowed to proceed, the
pH, and the reaction temperature.
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-14-
In certain embodiments, e.g. where the neutral yeast p-D-galactoside
galactohydrolase is derived from a Kluyveromyces species, it may be
necessary to add potassium and magnesium for enzyme activity. In
embodiments where the pH must be adjusted up to 5.5 or higher, e.g. where
the pH of the intermediate aqueous solution has been adjusted to about 2.0 or
zero or less to deactivate the acid fungal lactase, the pH and salt can be
adjusted using potassium, magnesium chloride, and citric acid.
ENZECOTM Lactase NL 2.5X has a pH optimum of about 6 to about 7.
Accordingly, the skilled person will understand that it may be necessary to
adjust the pH of the intermediate aqueous solution between 6 and 7.5 to
facilitate the activity of the neutral yeast lactase. In particular
embodiments,
adjusting pH of the intermediate solution with potassium hydroxide,
magnesium chloride and citric acid involves adjusting the pH to about 6.8.
Such pH adjustments can lead to turbidity of the mixture, which can plug
downstream separation equipment. However, this turbidity can largely be
avoided by adding the salts in a specific sequence. More particularly,
adjusting the pH of the intermediate aqueous solution to the desired pH and
salt concentration by sequentially adding the potassium hydroxide,
magnesium chloride and citric acid can avoid turbidity. More particularly,
sequentially adding potassium hydroxide, magnesium chloride and citric acid
to the intermediate aqueous solution in the following amount and order can
largely avoid turbidity:
= adding potassium hydroxide to arrive at a pH of about 9.2;
= adding magnesium chloride to arrive at a pH of about 9.1; and
= adding citric acid to a pH of about 6.8.
The temperature of the intermediate aqueous solution is adjusted to between
30 and 45 C prior to addition of the neutral yeast lactase. However, the
skilled
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-15-
person will understand that while temperature may be adjusted for optimal
enzyme activity, the yeast lactase may perform at a much slower rate outside
this range, e.g. between about 4.0 and about 50.0 C. In particular
embodiments disclosed herein, the temperature of the intermediate aqueous
solution is adjusted to about 36.5 C for incubation with the neutral yeast
lactase. As with the acid fungal lactase, the reaction time will depend on
temperature, pH, lactase concentration, and initial concentration of lactose
in
the intermediate aqueous solution. Again, the reaction rate can be increased
if
enzyme cost is not a concern. Alternatively, the reactions may be run more
slowly to save on the cost of enzyme.
The intermediate aqueous solution is incubated with the neutral yeast lactase
to produce a final aqueous solution in which the concentration of lactose is
between zero and about 20% of the initial concentration of lactose in the
initial
aqueous solution. In some embodiments, the intermediate aqueous solution
is incubated with the neutral yeast lactase until a final aqueous solution
comprising 23.5 % to 25% DP2 sugar (w/w) of total sugar in the final aqueous
solution is achieved.
Deactivation of the Yeast Lactase
Once a final concentration of between zero and 20% of the initial
concentration of lactose and the initial aqueous solution has been achieved,
the neutral yeast lactase may be deactivated. In some embodiments,
deactivating the neutral yeast lactase involves adjusting the pH of the final
aqueous solution to about pH 5.5, at or below which pH the enzyme
effectively has no activity. In addition to adjusting the pH to 5.5, or as an
alternative to adjusting pH to 5.5, deactivating the yeast lactase may involve
incubating the final aqueous solution at 72 C. The necessity of the pH
adjustment step may depend on how quickly the final aqueous solution can be
heated, and how quickly the reaction is proceeding prior to such heat
treatment. If heating can be accomplished quickly enough so that there is no
change in sugar composition (e.g. hydrolysis of GOS) while the final aqueous
solution is being heated, then a pH adjustment may be unnecessary. On the
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-16-
other hand, the skilled person will appreciate that it may be unnecessary to
heat treat the final aqueous solution to deactivate the neutral yeast lactase
if
pH is used to deactivate the reaction, the reaction rate is very slow, or the
enzyme a little to no activity remaining.
Separation
Chromatography may then be used to remove the enzymes, stabilizing
agents, glucose and galactose from the final aqueous solution to produce a
GOS-enriched solution. Ion
exchange chromatography may be initially
carried out on the final aqueous solution to remove the lactase enzymes,
cations, anions, and components contributing to color.
After ion exchange, the further separation may be conducted to partially
remove glucose and galactose and enrich the GOS fraction. The skilled
person will be aware of the standard methods that may be available, including
ion exchange, filtration, chromatographic separation (SMB), or additional
fermentation reactions.
For example, simulated moving bed chromatography may be used to enrich
the GOS in the GOS syrup from about 40% w/w of total carbohydrate in the
final aqueous solution to greater than 60% w/w of total carbohydrates after
separation.
GOS Products
The composition of different GOS species in a GOS syrup is unpredictable
and will depend on the specific lactase with which lactose solution is
incubated, the concentration of lactose, and the concentration of lactose.
Accordingly, the skilled person will appreciate that the GOS syrups disclosed
herein have a unique balance of di- (DP2), tri- (DP3), tetra- (DP4), penta-
(DP5) and higher GOS. Accordingly, this disclosure also relates to GOS
syrups with novel GOS balances that are produced according to methods
disclosed herein.
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-17-
Accordingly, this disclosure further relates to use of the combination of a
first
p-D-galactoside galactohydrolase derived from an Aspergillus oryzae with a
second p-D-galactoside galactohydrolase derive from a Kluyveromyces lactis
in the preparation of GOS syrup from an aqueous solution comprising lactose.
The GOS syrup may comprise at least 40% GOS w/w of total carbohydrate in
the GOS syrup. The use involves incubation of the aqueous solution with the
first 13-D-galactoside galactohydrolase followed by incubation with the second
p-D-galactoside galactohydrolase.
EXAMPLE 1
An aqueous solution of edible lactose with a starting concentration of 45 Bx
as adjusted to pH=5 using hydrochloric acid and equilibrated to 53.5 C. p-D-
galactoside galactohydrolase derived from Aspergillus oryzae (ENZECOTM
Fungal Lactase Concentrate from Enzyme Development Company) was
added to the aqueous solution to a concentration of 280 LU per gram of
lactose in the aqueous solution. The initial aqueous solution was incubated
with the p-D-galactoside galactohydrolase derived from Aspergillus oryzae for
195 minutes under constant agitation. Samples of the aqueous solution were
taken at 1 min, 2.5 min, 5 min, 10 min, 15 min, 20 min, 25 min, 30 min, 40
min, 50 min, 60, min, 75 min, 90 min, 120 min, and 195 min. The composition
of the carbohydrate fractions of the aqueous solution at the different time
points are indicated in Table 1.
EXAMPLE 2
4.5 kg of edible lactose was suspended in 5.5 kg of water. The temperature
of the suspension was brought to above 90 C under constant agitation until
the lactose was completely dissolved to produce an initial aqueous solution.
The pH of the initial aqueous solution was adjusted to about 4.5 using
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-18-
hydrochloric acid. The temperature of the initial aqueous solution was
equilibrated to 55 C. p-D-
galactoside galactohydrolase derived from
Aspergillus oryzae (ENZECOTm Fungal Lactase Concentrate from Enzyme
Development Company) was added to the initial aqueous solution to a
concentration of 20 LAU/g lactose. The initial aqueous solution was incubated
with the 13-D-galactoside galactohydrolase derived from Aspergillus oryzae for
6 hours under constant agitation. The p-D-galactoside galactohydrolase was
then deactivated by adjusting the pH to about 2.0 with HCI.
The resulting intermediate solution comprising of GOS, glucose, galactose,
and unreacted lactose, was analyzed by HPLC to ensure that the lactose
concentration was reduced to less than 60% of the initial concentration of
lactose in the initial aqueous solution (see Figure 1, Table 2).
The pH of the intermediate solution was adjusted to about pH 8 with 50%
KOH. The pH of the intermediate solution was then adjusted to 6.75 with a
salt solution comprising of 3.72% w/w citric acid, 6.01% w/w magnesium
chloride hexahydrate, and 15.55% w/w dipotassium hydrogen phosphate. 13-
D-galactoside galactohydrolase derived from Kluyveromyces lactis
(ENZECOTm Lactase NL 2.5x from Enzyme Development Company) was then
added at a dosage of 8.8 LAU/g lactose. The intermediate solution was
incubated with p-D-galactoside galactohydrolase derived from Kluyveromyces
lactis for 10 hours under constant agitation. The p-D-
galactoside
galactohydrolase derived from Kluyveromyces lactis was then deactivated by
adjusting the pH to about 3.0 with HCI.
ao
Table 1. GOS produced using lactase (280 LU/g lactose) from Aspergillus Oryzae
(Enzeco Fungal lactase) at starting lactose of 45 BRIX,
T=53.5C, pH =5.
Time
(min) 1 2.5 5 10 15 20 25 30 40 50 60
75 90 120 195
DP5+
0.063 0.286 0.790 1.601 2.250 2.634 2.877 3.009 3.155
3.134 3.001 2.867 2.671 2.210 1.320
DP4
1.143 2.594 4.288 5.821 6.449 6.605 6.669 6.522 6.299
6.051 5.649 5.247 4.917 4.214 2.957
DP3
12.532 16.610 18.862 19.508 18.954 18.265 17.792 17.223
16.197 15.652 14.742 13.831 13.053 11.815 9.206
Lactose 78.856 69.258 57.818 48.774 42.769 38.460 35.558 33.051 28.909 26.122
23.476 20.830 18.760 15.493 10.976
0P2
0.532 0.382 2.339 2.913 4.257 5.630 6.342 7.100 8.657
9.674 10.650 11.626 12.136 13.289 13.673
Glucose
5.881 9.039 12.695 16.687 19.316 21.209 22.778 24.148
26.257 27.719 29.345 30.970 32.289 34.402 38.059
Galactose 0.993 1.830 3.207 4.697 6.004 7.197 7.984 8.947 10.526 11.648 13.138
14.629 16.174 18.578 23.810
01
TOTAL
GOS
14.271 19.872 26.280 29.843 31.910 33.134 33.680 33.854
34.308 34.510 34.041 33.572 32.777 31.528 27.155
CA 02992497 2018-01-15
WO 2017/120678 PCT/CA2017/050042
-20-
Table 2. % Composition of sugars in GOS mixture following primary
transgalactosylation of lactose using fungal p-galactosidase from
Aspergillus oryzae
carbohydrate % w/w of the total carbohydrate
DP6 0.495
DP5 1.884
DP4 6.505
DP3 19.050
DP2 4.005
Lactose 42.348
Glucose 19.634
Galactose 6.079
TOTAL GOS 31.939
The resulting final aqueous solution comprising of GOS, glucose, galactose
and unreacted lactose, was analyzed by HPLC to ensure that the lactose
concentration was to 10% or less than the initial concentration of lactose in
the initial solution (see Figure 2, Table 3).
Table 3. %Composition of sugars in GOS mixture following secondary
transgalactosylation of lactose using yeast 13-galactosidase from
Kluyveronnyces
carbohydrate % w/w of the total carbohydrate
DP6+ 0.435
DP5 1.908
DP4 6.742
DP3 18.580
DP2 12.842
Lactose 8.733
Glucose 33.67
Galactose 17.082
TOTAL GOS 40.506
EXAMPLE 3
Demineralized, deproteinized, ultrafiltered milk permeate was evaporated to
35 Bx, and incubated with p-D-galactoside galactohydrolases derived from
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-21-
Aspergillus oryzae and Kluyveromyces lacti as described in Example 2. The
composition of sugars in the GOS mixture following two-stage
transgalactosylation of lactose from ultrafiltered milk permeate is shown in
Table 4.
Table 4. % Composition of sugars in GOS mixture following two-stage
transgalactosylation of lactose from ultrafiltered milk permeate using
yeast p-galactosidase from Aspergillus Oryzae and Kluyveromyces
Lactis.
Name % w/w of the total carbohydrate
DP5+ 1.328
DP4 6.094
DP3 18.143
DP2 15.475
Lactose 8.523
Glucose 33.973
Galactose 16.464
TOTAL GOS 41.040
EXAMPLE 4
27.4 kg of edible lactose was suspended in 20.8 kg of water to produce a
solution of 54 Bx. The temperature of the suspension was brought to above
95 C under constant agitation until the lactose was completely dissolved to
produce an initial aqueous solution. The pH of the initial aqueous solution
was 5.4. The temperature of the initial aqueous solution was equilibrated to
58.5 C. p-D-galactoside galactohydrolase derived from Aspergillus oryzae
(ENZECOTm Fungal Lactase Concentrate from Enzyme Development
Company) was added to the initial aqueous solution to a concentration of 277
LU/g lactose. The initial aqueous solution was incubated with the p-D-
galactoside galactohydrolase derived from Aspergillus oryzae for 15 minutes
under constant agitation. The p-D-galactoside galactohydrolase was then
deactivated by adjusting the pH to about 2.0 with HCI.
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-22-
The resulting intermediate solution comprising of GOS, glucose, galactose,
and unreacted lactose, was analyzed by HPLC to ensure that the lactose
concentration was reduced to less than 60% of the initial concentration of
lactose in the initial aqueous solution (see Table 5).
The intermediate solution was diluted to 50BRIX. The pH was adjusted to
about pH 9.3 with 50% KOH. Magnesium chloride hexahydrate (25g) was
added and the pH adjusted to 6.80 using 50% citric acid. p-D-galactoside
galactohydrolase derived from Kluyveromyces lactis (ENZECOTM Lactase NL
2.5x from Enzyme Development Company) was then added at a dosage of
40.4 LU/g lactose. The temperature of the solution was adjusted to 40 C.
The intermediate solution was incubated with p-D-galactoside
galactohydrolase derived from Kluyveromyces lactis for 100 minutes under
constant agitation. The p-D-
galactoside galactohydrolase derived from
Kluyveromyces lactis was then deactivated by adjusting the pH to about 3.0
with HCI.
Table 5. % Composition of sugars in GOS mixture following primary
transgalactosylation of lactose using fungal 13-galactosidase from
Aspergillus oryzae
Name % w/w of the total carbohydrate
DP6 0.397
DP5 1.812
DP4 6.795
DP3 19.773
DP2 3.216
Lactose 43.655
Glucose 18.875
Galactose 5.478
TOTAL GOS 31.595
The resulting final aqueous solution comprising of GOS, glucose, galactose
and unreacted lactose, was analyzed by HPLC (see Table 6).
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-23-
Table 6. % Composition of sugars in GOS mixture following secondary
transgalactosylation of lactose using yeast I3-galactosidase from
Kluyveromyces
Name % w/w of the total carbohydrate
DP6+ 0.380
DP5 1.840
DP4 6.892
DP3 19.302
DP2 13.403
Lactose 8.691
Glucose 32.573
Galactose 16.919
TOTAL GOS 41.817
EXAMPLE 5
Edible crystalline lactose (Lynn Proteins, Inc., Granton, WI) was dissolved in
water at 90 C to a final concentration of 45913x to produce an initial aqueous
solution of lactose. The temperature of the initial lactose solution was
equilibrated to about 53.5C, and the pH was adjusted to between 4.5 and 5.0
using HCI. Fungal p-D-galactoside galactohydrolase derived from Aspergfflus
oryzae (ENZECOTM Fungal Lactase Concentrate from Enzyme Development
Company) was then added to the initial aqueous solution to a concentration of
5.6 LU/g of lactose. The solution was incubated 17 hours under constant
agitation to produce an intermediate aqueous solution comprising lactose at a
concentration about 40% of the initial concentration of lactose in the initial
aqueous solution. The fungal p-D-galactoside galactohydrolase was then
deactivated by adjusting the pH to about 2.0 with HCI using a 15% w/w
aqueous solution of HCI. After 60 minutes of steady agitation, a 50% w/w
solution of KOH was slowly added to the intermediate aqueous solution,
thereby adjusting the pH to about 9.30. A 25% w/v solution of magnesium
chloride hexahydrate was then added to a concentration of 0.16% w/w of the
intermediate aqueous solution, thereby adjusting the pH to about 9.21. Then,
a 50% solution of citric acid was slowly added to the intermediate aqueous
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-24-
solution until the pH reached about 6.8. The intermediate aqueous solution
was then equilibrated to 36.5 C. Yeast pi-D-galactoside galactohydrolase
derived from Kluyveromyces lactis (ENZECOTm Lactase NL 2.5x from Enzyme
Development Company) was then added to a concentration of 4.7 LU/g of
lactose to the intermediate aqueous solution. The intermediate aqueous
solution was incubated for 17h under steady agitation to produce a final
aqueous solution comprising lactose at a concentration less than 20% of the
lactose concentration in the initial aqueous solution. The pH of the final
aqueous solution was adjusted to pH 5.5 with citric acid to deactivate the 13-
o-
galactoside galactohydrolase derived from Kluyveromyces lactis. The final
aqueous solution was then heat treated at 72 C for 15 seconds. The
carbohydrate composition of the final aqueous solution from five trials is
provided in Table 7.
The final aqueous solution was subjected to ion exchange purification to
remove the salts, lactase enzymes and color components. After ion
exchange, the partially purified solution was subjected to a purification step
to
enrich the GOS fraction. The carbohydrate composition of the GOS syrup final
aqueous solution from five trials is provided in Table 7.
Table 7. Carbohydrate composition of GOS syrup produced using a
combination of lactases from Aspergillus oryzae and Kluyveromyces lactis
prior to chromatographic separation.
% w/w of the total carbohydrate
Name Stage 1 (t=17h) Stage 2 (t=17h) Enrichment
DP6 0.450 0.396 0.683
DP5 1.973 1.743 2.946
DP4 6.743 6.638 11.133
DP3 18.770 18.851 32.972
DP2 4.069 12.652 18.216
Lactose 40.792 8.830 14.891
DP2 + Lactose 44.861 21.482 88.107
Glucose 20.581 33.526 17.346
Galactose 6.622 17.364 1.814
TOTAL GOS 32.006 40.280 65.950
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-25-
EXAM PL E 6
Edible crystalline lactose (Lynn Proteins, Inc., Granton, WI) was dissolved in
water at 95 C to a final concentration of 53 Bx to produce an initial aqueous
solution of lactose. The temperature of the initial lactose solution was
equilibrated to about 55-56.5 C, and the pH was adjusted to between 4.5 and
5.5. Fungal p-D-galactoside galactohydrolase derived from Aspergillus oryzae
(ENZECOTm Fungal Lactase Concentrate from Enzyme Development
Company) was then added to the initial aqueous solution to a concentration of
5.8 LU/g of lactose. The solution was incubated 11 hours under constant
agitation to produce an intermediate aqueous solution comprising lactose at a
concentration about 40% of the initial concentration of lactose in the initial
aqueous solution, and DP2 sugar at 49% to 52% of total sugar. Thus,
increasing the initial lactose concentration and enzyme concentration reduced
the required reaction time from 17 h to 11 h. The fungal p-D-galactoside
galactohydrolase was then deactivated by adjusting the pH to about 2.0 with
HCI using a 15% w/w aqueous solution of HCI. a 20% w/w solution of KOH
was slowly added to the intermediate aqueous solution, thereby adjusting the
pH to about 9.30. A 25% w/v solution of magnesium chloride hexahydrate was
then added to a concentration of 0.16% w/w of the intermediate aqueous
solution, thereby adjusting the pH to about 9.21, and essential ions were
added to the second enzymatic reaction. Then, a 20% solution of citric acid
was slowly added to the intermediate aqueous solution until the pH reached
about 6.8. The intermediate aqueous solution was then equilibrated to 36.5 C.
Yeast p-D-galactoside galactohydrolase derived from Kluyveromyces lactis
(ENZECOTm Lactase NL 2.5x from Enzyme Development Company) was then
added to a concentration of 4.4 LU/g of lactose to the intermediate aqueous
solution. The intermediate aqueous solution was incubated for 17h under
steady agitation to produce a final aqueous solution comprising lactose at a
concentration less than 20% of the lactose concentration in the initial
aqueous
solution, and DP2 sugar at 23.5% to 25% of total sugar). The final aqueous
solution was then heat treated at 72 C for 15 seconds to deactivate the p-D-
galactoside galactohydrolase derived from Kluyveromyces lactis. The
CA 02992497 2018-01-15
WO 2017/120678
PCT/CA2017/050042
-26-
carbohydrate composition of the final aqueous solution from ten trials is
provided in Table 8.
The final aqueous solution was subjected to ion exchange purification to
remove the salts, lactase enzymes and color components. After ion
exchange, the partially purified solution was subjected to a purification step
to
enrich the GOS fraction. The carbohydrate composition of the GOS syrup final
aqueous solution from five trials is provided in Table 8.
Table 8 Carbohydrate composition of GOS syrup produced using a
combination of lactases from Aspergillus oryzae and Kluyveromyces lactis
prior to chromatographic separation.
% w/w of the total carbohydrate
Name Stage 1 (t=1 1 h) Stage 2 (t=1 7h)
Enrichment
DP6 0.172 0.051 0.187 0.044 0.278
0.033
DP5 1.407 0.085 1.574 0.112 2.237
0.136
DP4 6.165 0.144 6.851 0.254 10.126
0.433
DP3 19.816 + 0.691 20.042 0.524 30.882
0.748
DP2 with lactose 50.564 + 0.504 23.821 0.783 35.460
0.838
Glucose 17.247 + 0.553 32.108 0.640 18.624
0.939
Galactose 4.623 0.565 15.416 0.537 2.393
0.607
TOTAL GOS 64.531 1.463
While specific embodiments of the invention have been described and
illustrated, such embodiments should be considered illustrative of the
invention only and not as limiting the invention as construed in accordance
with the accompanying claims.