Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-1-
APPARATUS AND METHOD FOR RECOVERY OF METAL USING MULTI-
COMPARTMENT ELECTROLYTIC CELL
[0001] This invention was made and reduced to practice
in part with US government support under National
Institutes of Health (NTH) Small Business Innovation
Research (SBIR) grant 1 R43 E520096-01 AND Department of
Energy (DOE) Small Business Innovation Research (SBIR)
grant DE-5C0006181. The US government has certain rights
in the invention.
FIELD OF THE INVENTION
[0002] The invention relates to an apparatus and a
method for electrochemical modification of concentrations
of constituents of liquid streams which contain organic
and/or inorganic components. More precisely, the invention
is concerned with an electrolytic cell technology with
potentials to modification of concentrations of the
components.
Date Recue/Date Received 2020-12-02
CA 2885437 2018-02-15
- 2 -
BACKGROUND OF THE INVENTION
[0003] Contamination of liquid streams with various
organic and inorganic constituents may represent an
environmental problem affecting environment quality and
represents significant threat to human health and safety.
For example, heavy metals contaminations of aquatic
environments may arise from commercial mining and metal
extraction processes, surfaces modification and protection
processes, or communal and industrial waste sites resulting
from a variety of active or defunct industrial fabrication
and manufacturing activities. Similarly, significant
organic water pollutants, like aliphatic, aromatic, or
halogenated hydrocarbons and phenols may be associated with
oil exploration, extraction and refining, chemicals
production, or large-scale farming and food processing.
[0004] In addition to potentials for significant
environmental damage, affected liquid streams my represent
dilute sources of desirable raw materials like heavy
metals, metal oxides, inorganic salts, and other compounds.
For example, the Berkeley Mine Pit in Butte, Montana alone
represents an estimated 30 billion gallons of acid mine
drainage which at one time contained -180 ppm of copper
CA 02885437 2015-03-20
- 3 -
along with other metals which could yield up to 22,000 tons
of pure copper by use of a small treatment facility.
[0005] An economically relevant group of prior art
methods of removal of heavy metal ions from liquid
solutions is based on chemical precipitation. This process
is likely burdened by complexity, high cost, clear
preference for extremely large facilities and high-volume
operations, and efficiency decrease with decrease in
concentration of pollutants. Additional disadvantages may
concern resulting byproduct of precipitated sludge which
may become a concentrated yet mixed contaminant source of
the toxins in the source material. The sludge may mandate
further processing and costly long term disposal as a
highly toxic waste. Many similar disadvantages may burden
alternative heavy ion removal methods that may incorporate:
filtration, ion exchange, foam generation and separation,
reverse osmosis, or combinations of listed processes.
[0006] In contrast, the extraction technologies enabled
by several aspects of the current invention may be adapted
to alleviate at least some of the above considerations.
Additional features of the current invention, for example,
may contribute to the feasibility of modifying prior art
electrowinning technology so that it can be used to
CA 02885437 2015-03-20
, 3
- 4 -
economically concentrate copper generated in low-grade
process streams instead of simply removing it. In general,
the disclosed embodiments of the copper extraction
technology may prepare a process stream so the customer can
produce new copper from currently inaccessible sources with
existing in-place processing infrastructure, equipment, and
processes.
[0007] The present invention may provide some innovative
features for unlocking this vast and vitally needed
resource. Typical mines contain significant amounts of
their copper in such unviable ores. This invention may
allow the use of this "waste" ore and thereby increase
average heap leach mine output by 25% and thus globally
yield 3 Billion lbs/yr of newly recoverable copper.
[0008] Furthermore, additional features of embodiments
of the current invention may allow for practical metal
recovery from: Acid Rock Drainage (ARD), heavy metal and
radionuclide contaminated sites, and metal contaminated
industrial effluents such as electrowinning, plating plant,
pickling operations, and circuit board manufacture
(etching) discharges.
CA 02885437 2015-03-20
- 5 -
[0009] In addition, different embodiments of the current
invention may be applicable and pertinent to commercial and
municipal processes where potential contaminants may be
reprocessed in parallel or in immediate sequence with
processes that may generate such materials to start with.
Even further, methods and apparatus of the current
invention may achieve the above functions in an essentially
integrated manner, frequently using at least one common
treatment loop to simultaneously refine the desired
products, generate materials and compounds that may be
reused in the subsequent performances of the process by the
disclosed apparatus, and generate essentially non-polluting
byproducts.
[0010] Finally, by application of highly integrated
multifunctional devices and processes, the components of
the current invention may achieve desirable results
utilizing optimized quantities of components, raw
materials, ingredients, and required energy; thus
approaching optimized economic results.
SUMMARY OF THE INVENTION
[0011] A method and an apparatus for electrochemical
modification of liquid streams employing an electrolytic
- 6 -
cell which utilizes an anode compartment defined by an
anode structure where oxidation is effected, containing a
liquid electrolyte anolyte, and a cathode compartment
defined by a cathode structure where reduction is effected
containing a liquid electrolyte catholyte. In addition at
least one additional compartment has been arranged at least
partially between the anode compartment and the cathode
compartment and separated from the anode compartment and
the cathode compartment by a separator structure arranged
to support ionic conduction of current between the anode
structure and the cathode structure. Also, a system for
conducting unidirectional electric current provides a
unidirectional current flow supported by the liquid
electrolytes from the anode structure through the separator
structure and into the catholyte and to the cathode
structure have been provided.
[0012] The separator
structure incorporates at least one
ion conductive membrane positioned at least partially
between the anode compartment and the at least one
additional compartment, and arranged to conduct a plurality
of cations while impeding transport of at least one
selection of anions, and at least another selective ion
conductive membrane positioned at least partially between
the cathode compartment and the at least one additional
CA 2885437 2018-05-17
- 7 -
compartment, and arranged to conduct at least another
selection of anions while impeding transport of the
plurality of cations.
[0012a] We further disclose an apparatus for
electrochemical modification of liquid streams employing at
least one electrolytic cell, which comprises:
an anode compartment defined by an anode structure
where oxidation is effected, containing a liquid
electrolyte anolyte;
a cathode compartment defined by a cathode
structure where reduction is effected containing a liquid
electrolyte catholyte;
at least one additional compartment arranged at
least partially between the anode compartment and the
cathode compartment and separated from the anode
compartment and the cathode compartment by a separator
structure arranged to support ionic conduction of current
between the anode structure and the cathode structure; and
CA 2885437 2018-05-17
- 7a -
a system for conducting unidirectional electrolyte
supported electric current flow between the anode structure
and into the anolyte and through the separator structure
and into the catholyte and to the cathode structure.
[0012b1 The separator structure incorporates at least one
ion conductive membrane positioned at least partially
between the anode compartment and the at least one
additional compartment, and arranged to preferentially
conduct a plurality of first ions while impeding transport
of at least one selection of second ions, and at least
another ion conductive membrane positioned at least
partially between the cathode compartment and the at least
one additional compartment, and arranged to preferentially
conduct at least another selection of third ions while
impeding transport of the plurality of first ions.
[0012c] Furthermore, the first and second ions differ in
charge sign and the first and third ions differ in charge
sign.
CA 2885437 2018-05-17
- 7b -
[0012d] We also disclose that the first ion may be a cation
and the second ion may be an anion and the third ion may be
another anion. Furthermore, the first ion may be a
hydrogen-like cation.
[0012e] We further
disclose a method for electrochemical
modification of liquid streams employing at least one
electrolytic cell, which comprises the steps of:
(a) providing the at least one electrolytic cell
incorporating an anode compartment defined by an anode
structure where oxidation is effected, containing a liquid
electrolyte anolyte, a cathode compartment defined by a
cathode structure where reduction is effected, containing a
liquid electrolyte catholyte, at least one additional
compartment arranged at least partially between the anode
compartment and the cathode compartment and separated from
the anode compartment and the cathode compartment by a
separator structure arranged to support ionic conduction of
current between the anode structure and the cathode
structure and a particulate manipulation system arranged to
manipulate particulates motion such after participating in
the target redox reactions, the anode and the cathode
CA 2885437 2018-05-17
- 7c -
particles are separated from the respective anolyte and
catholyte, and controllably passed to the cathode
compartment and the anode compartment respectively, and a
system for conducting unidirectional electrolyte supported
electric current flow between the anode structure, into the
anolyte, and through the separator structure and into the
catholyte and to the cathode structure; wherein the
separator structure incorporates at least one ion
conductive membrane positioned at least partially between
the anode compartment and the at least one additional
compartment, and arranged to preferentially conduct a
plurality of first ions while impeding transport of at
least one selection of second ions, and at least another
ion conductive membrane positioned at least partially
between the cathode compartment and the at least one
additional compartment, and arranged to preferentially
conduct at least another selection of third ions while
impeding transport of the plurality of the first ions;
furthermore, the first and second ions differ in charge
sign and the first and third ions differ in charge sign;
CA 2885437 2018-05-17
CA 2885437 2018-02-15
- 7d -
(b) providing an amount of raw materials and performing
leaching to extract a leachate in a form of a pregnant
leach solution;
(c) performing refining of the pregnant leach solution to
extract desired metals and generate a raffinate;
(d) inputting an Acid Rock Drainage or a leachate product
into the cathode compartment and the raffinate into the at
least one additional compartment and conduct an
unidirectional electric current flow between the anode
structure to the cathode structure; and
(e) redirecting an elevated acidity output from the at
least one additional compartment onto the amount of raw
material until predetermined quantities of the desired
metals have been extracted.
CA 2885437 2018-02-15
- 7e -
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] The above and other embodiments, features, and
aspects of the present invention are considered in more
detail in relation to the following description of
embodiments shown in the accompanying drawings, in which:
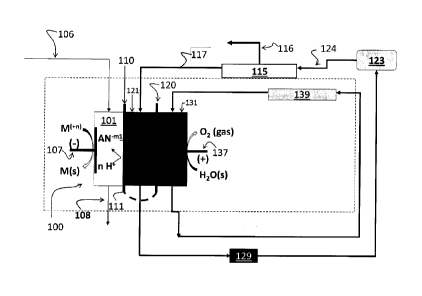
[0014] FIG. 1. is a schematic view of devices and
processes of some embodiments in accordance with the
current invention.
[0015] FIG. 2. is a schematic view of devices and
processes of different embodiments in accordance with the
current invention.
[0016] FIG. 3. is a schematic view of devices and
processes of other different embodiments in accordance with
the current invention.
CA 02885437 2015-03-20
- 8 -
DETAILED DESCRIPTION OF THE INVENTION
[0017] The invention summarized above may be better
understood by referring to the following description, which
should be read in conjunction with the accompanying
drawings. This description of an embodiment, set out below
to enable one to build and use an implementation of the
invention, is not intended to limit the invention, but to
serve as a particular example thereof. Those skilled in the
art should appreciate that they may readily use the
conception and specific embodiments disclosed as a basis
for modifying or designing other methods and systems for
carrying out the same purposes of the present invention.
Those skilled in the art should also realize that such
equivalent assemblies do not depart from the spirit and
scope of the invention in its broadest form. Embodiments
of this Instant invention can be of planar, circular, and
concentric tubular or other configurations containing two
or more separate electrolyte compartments as required to
address different application needs.
[0018] One embodiment of the instant invention is
illustrated in Fig. 1.
- 9 -
[0019] In specific
embodiments of the current invention,
at least one additional compartment 121 may be added
nominally between (at least partially) the cathode and
anode compartments 101 and 131 respectively, and separated
by at least one separator structure 111 arranged to prevent
bulk mixing of the contents of the separated compartments
101, 121, and 131. In some embodiments, the separator
structure 111 may include a pair of ion conductive elements
arranged to allow transport of desired ion species between
the compartments 101, 121, and 131. In one group of
embodiments the separator structure 111 may incorporate ion
conductive membranes 110 and 120 at least partially between
and in direct contact with the contents of the compartments
101 and 121, and compartments 121 and 131 (i.e. contactly
separating compartments 101, 121, and 131). The ion
conductive membranes may incorporate ion conductive
channels such that specific ions may be transported between
compartments with fluxes depending on specific embodiment
parameters including applied voltage, ion concentrations,
temperature, ion mobility, and dimensions and surface
properties of channels etc. The parameters may be arranged
such that desired Anions are preferably transported from
the cathode compartment 101 into the at least one
additional compartment 121, while desired Cations may be
CA 2885437 2018-05-17
CA 2885437 2018-02-15
- 10 -
transported from the anode compartment 131 into the at
least one additional compartment 121.
[0020] A particular combination of the electrical fields
for the cell operation in conjunction with the selective
nature of the separating membranes may allow one to
separate and transfer into the at least one additional
compartment 121 ions of interest so that they may be
concentrated in the added compartment in the manner
comparable to electrodialysis. One feature of such
modified cell may be to gain practical utility for several
industrially important applications.
[0021] In the Fig. 1 schematically illustrated example,
at least one electrolytic cell 100 having at least three
compartments with at least two separate electrolyte flows
separated by at least two ion conductive membranes 110 and
120 respectively. The at least one cathode compartment 101
input 106 may depend on the specific circumstances of the
application and may include any number of liquid (aqueous
and nonaqueous) streams containing dilute reduction targets
such as metals of suitable redox activity. A cathode
structure 107 may be conventional (stationary) assembly or
may, in different embodiments, incorporate a moving bed of
CA 2885437 2018-02-15
- 11 -
conductive particulates for which numerous compositions may
be appropriate.
[0022] Several metals indicative for some aqueous
solutions of interest as the input 106, may include (but
not be limited by): copper, iron, nickel, cobalt, cadmium,
zinc, indium, gold, platinum, palladium, silver, mercury,
tin, and rhenium. Other metals "M(s)" or metal Cations
"M"or metal containing ion complexes could be addressed
in applications where one of the goals my be to reduce the
metal's oxidation state (e.g. from +n to +(n-1),
and not necessarily to plate the metal out. The
solutions' pH may be in the range from strongly acidic to
strongly alkaline.
[0023] In particular embodiments, the input 106 may
include Acid Rock Drainage (ARD) containing, for example,
high sulfate acidic solutions including mixtures of
dissolved and often dilute metals, as a result of natural
processes attacking exposed sulfide containing rock (ore).
The cathode compartment output 108 then may have the
concentrations of the target metal ion lowered (this may
allow for transformation of the species from one oxidation
state to another without actual removal of the target metal
ion from solution) and the sulfate concentration lowered.
- 12 -
In another embodiment, the input 106 may include leachate
product.
[0024] Depending upon different embodiments, at least
one anode structure 137 of at least one anode compartment
131 may either utilize a conventional geometrically stable
DSA electrode or may incorporate moving beds of
particulates, for example, using particulates transferred
to/from the cathode compartment 101.
[0024a] Conveyance
systems for placing the anode and/or
cathode particulates in motion within the respective anode
and/or cathode structures are disclosed, for example, in
U.S. 8,545,692 (James et al.). The
particulates may thus
be arranged and driven to move essentially independently of
the electrolyte flow, as disclosed for example in James et
al., whereby at least a portion of the particulates may be
separated from the respective anolyte and catholyte fluids
and at least another portion of the particulates are
transferred between the cathode and anode particulates beds
in a continuous loop after participating in a redox
reaction.
CA 2885437 2018-05-17
- 12a -
[0025] At least one additional compartment 121 may be
arranged at least partially between the at least one anode
compartment 131 and the at least one cathode compartment
101, and separated from the anode compartment and the
cathode compartment by the separator structure 111,
incorporating at least two ion conductive membranes 110 and
120, arranged to support ionic conduction of current
between the anode structure 137 and the cathode structure
107, but to restrict transport of selected Anions AN-rn2 from
the anode compartment 131 into the at least one additional
compartment 121, and to restrict transport of Cations (nH)
from the at least one cathode compartment 101 into the at
least one additional compartment 131.
CA 2885437 2018-05-17
- 13 -
[0026] In some embodiments, the at least one ion
conductive membrane 110 may represent a selective ion
conductive membrane positioned at least partially between
the at least one cathode compartment 101 and at least one
additional compartment 121 (while being in direct contact
with the liquids contained in the compartments 101 and 121)
and arranged to selectively conduct specific Anions AN-ml- or
a composition of Anions as appropriate to the specific
embodiments, while impeding transport of cations such as
Hydrogen FP- or Hydrogen-like (e.g. "Hydronium-" (H20+),
"Zundel'"(H502), or "EigenTm") (H9oe) or others. In
contrast, the at least one ion conductive membrane 120 may
represent a selective ion conductive membrane at least
partially between the at least one anode compartment 131
and at least one additional compartment 121 (while being in
direct contact with the liquids contained in the
compartments 131 and 121) and arranged to conduct cations
such as Hydrogen H+ or Hydrogen-like cations or others while
restricting transport of specific Anions AN-m2 or a
composition of Anions as appropriate to the specific
embodiments. As a result, operations of the cell 100 may
result, inter alia, in gradual increase of concentrations
of AN-ml and H+ ions (and therefore the 1-1.1AN acid)in the at
least one additional compartment 121. Therefore, outputs
129 from the compartment 121, having elevated acidity, may
CA 2885437 2018-05-17
- 14 -
be used for other purposes as the particular groups of
embodiments may mandate or desire.
[0027] One embodiment may include processing of zinc -
where the particulate bed may be transferred into the
anolyte but not in electrical contact with the anode
structure 137. The anode structure may utilize a separate
DSA electrode for oxygen evolution (for example) from water
splitting (H20 4 4H+ + 02 + e-) and the zinc being
spontaneously stripped from the particulate electrode
[using an inert substrate like 316 SS] to generate
concentrated ZnSO4 in the anolyte). The water splitting on
the anode includes generation of protons which may
subsequently be separated from the background supporting
electrolyte (salt) via the proton selective Cation
conductive membrane 120. Membranes selective to other
Cations could also be used (for the appropriate
embodiments).
[0028] It may be noted that the supporting electrolyte
139 may include a number of common salts or mixtures
thereof. Different embodiments may include (but not be
limited to) all combinations of CA Cations where CA = Ht,
Nat, 1.<+, Li+, Cu+2, Ca+2, Mg+2, Mn+2,Ni+2, Fe+2, Fe+3, Al+3, and
NH4, and AN Anions where AN = Acetate, bromide, chloride,
Chlorate, cyanide, hydroxide, hypochlorite, iodate, iodide,
CA 2885437 2018-05-17
- 15 -
nitrate, oxalate, perchlorate, phosphate, hydrogen
phosphate, dihydrogen phosphate, bisulfate, and sulfate
plus those shown in the following Table I.
[0029] Table I.
Common Ion Chart
Positive Ions (Cations) Negative Ions (Anions)
Aluminum Al +3 Acetate C21-1302 /
CH3C00
Ammonium NH4 -3 Bromide Br
Barium Bit +2 Carbonate
CO3 -2
Cadmium Cd +2 Hydrogen Carbonate Ion /
BicarbonateHCO3.
Calcium Ca +2 Chlorate CI03 -
Chromium (II) Cr +2 Chloride Cl --
Chromium (III) Cr3-3 Chlorite C102
Cobalt (II) Co+2 Chromate Cr042----
Copper (1) Cu + Cyanide CN
Copper (II) Cu +2
Dichromate Cr,0- 2 _ ,
Hydrogen H + Fluoride F -
Hydronium H30 + Hydride H
Iron (II) Fe +2 Hydroxide OH
Iron (Ill) Fe +3 Hypochlorite CIO -
Lead (II) Ph +2 Iodate 103
Lead (IV) pb-t-4
Iodide I -
Lithium Li + Nitrate NO3 --
Magnesiummg +2
Nitride N 3
Manganese (II) Mn+2 Nitrite NO2 -
Mercury (I) Hg2 +2 Oxalate
C204 -1
Mercury (II) ug +2 Oxide 0 -2
Potassium K + Hydrogen Oxalate Ion HC204 -
Silver Ag + Perchlorate C104
Strontimn Sr +2
Permanganate Mn04
Sodium Na + Peroxide Ion 02 ' I
Tin (II) Sn -'2 Phosphate
PO4 --2
Tin (IV) Sn +4 Monohydrogen Phosphate HPO4 -1
Zinc Zn '2 Dihydmgen Phosphate 112PO4 '-
Silicate SiO3-2
Sulfate SO4 -2
Hydrogen Sulfate Ion / Bisulfate HSO4
1 ¨ mono 5 ¨ penta 9 ¨ nom Thiosulfate
S204 2
2¨ di 6¨ hexa 10¨ deca Sulfide s -2
3 ¨ tri 7 ¨ hepta Hydrogen Sulfide Ion / Bisulfide HS --
4 ¨ tetra 8 - octa Sulfite S03 2
Hydrogen Sulfite Ion / Bisulfite HS03-
CA 2885437 2018-05-17
- 16 -
[0030] As in the discussion of the above embodiments,
the at least one additional compartment 121 may be
delineated on either side by selective ion conductive
membranes 110 and 120 which allow selective ionic
conduction. In some embodiments, a simple physical barrier
such as a microporous membrane like DARAMIC available
commercially at least from the Daramic, LLC (a business
unit of Polypore, Inc. with headquarters in Charlotte,
North Carolina USA) could also be used but typically
achieves lower separation efficiency and resultant
concentration differentials than afforded by ion selective
membranes. Typically an Anion selective membrane may be
employed to separate the cathode compartment 101 and the
additional compartment 121. In such an embodiment, an
Anion conductive membrane exhibiting sulfate transport
selectivity may be used (several commercially available).
Many commercial varieties of Anion selective membranes
providing different characteristics and transport
properties are also available in alternative, and would be
suitable for use herein and in different embodiments. For
the anode compartment 131 /additional compartment 121
separation, a Cation selective membrane 120 may be used.
CA 2885437 2018-05-17
- 16a -
In some embodiments, a proton selective membrane like
DuPont's Nafion (or NAFION ) may be used, with a bipolar
variant being a possible choice due to its enhanced
suppression of back diffusion of Anions. Again, many other
commercial examples of Cation and proton selective Cation
selective membranes exist and may be suitable for use
herein and in other embodiments. The emerging polymer
membrane based fuel cell industry
CA 2885437 2018-05-17
- 17 -
represents a market of choice and a motivator for the
future R&D and may be a rich source of new and better
membranes for emerging and yet to emerge embodiments of the
current invention.
[0031] In the embodiments pertinent to the schematics
illustrated in Fig. 1, the outputs 129 may be used in an
extraction procedure wherein, a strong leachant solution
including the output 129 (having e.g. sulfuric acid) may be
passed through raw materials 123 (e.g. crushed ore) to
leach out target metal or metals from the raw materials
123. The resultant leachate 124 (pregnant leach solution
or PLS) may be subsequently processed in a reactor 115 to
remove the dissolved metals. Most of the target metals 116
may be removed, but a residual amount of target metal
remains in a weak solution called raffinate 117. It may be
noted that the leaching procedures may consumes the
leachant (again as acid contained in the output 129) and
fresh makeup leachant (here acid) may need to be added to
the residual raffinate before it is recycled back to leach
more raw materials 123.
[0032] It may be noted that in some embodiments, the
reactor 115 may be based on electrochemical principles and
incorporate electrolytic cells as recited above.
CA 2885437 2018-05-17
CA 02885437 2015-03-20
,
- 18 -
[0033] Regarding embodiments of the current invention
pertinent to the schematic illustration in Fig. 2, where it
may not be desirable to directly input solutions of
interest as the input 106 into the cathode compartment 101,
a portion of the raffinate 206 exiting the reactor 115 may
be inputted into the at least one cathode compartment 101.
The remaining portion of the raffinate 117 may, as before
(e.g. Fig. 1), be directed into the at least one additional
compartment 121 for recycling and further concentration of
the acid ingredients. It may be noted that the residual
metal in the raffinate 117 may not be lost for extraction
and is likely to be reacquired (e.g. plated as metal)
during the subsequent passages through the reactor 115
and/or the cathode compartment 101.
[0034] Furthermore, one may note that Fig. 2-illustrated
embodiments may include addition of further external
components 210 intended to improve devices and operations
acting, for example, as supporting solvents (aqueous or
non-aqueous), detergents, lubricants, emulgators,
coagulats, surfactants, buffers, corrosion inhibitors,
and/or combinations of the above functions.
CA 2885437 2018-02-15
- 19 -
[0035] Regarding embodiments of the current invention
pertinent to the schematic illustration in Fig. 3, where it
may not be sufficient amount (or flow) of the input 106
(e.g. ARD), the input 106 may vary in time (seasonally or
in response to meteorological conditions), or/and may be
suboptimal to introduce only the input 106 directly into
the at least one cathode compartment 101, the input 106 may
be in-mixed with the portion 306 of the raffinate, in-mixed
directly into the reactor 115, and/or co-applied to the raw
materials 123. It may be noted that, as discussed above
regarding the Fig.2, the metal content in the input 106
exiting through the portion 117 of the raffinate may not be
lost for extraction and is likely to be reacquired (e.g.
plated as metal) during the subsequent passages through the
reactor 115 and/or the cathode compartment 101.
[0036] Processes of controlled removal of products of
electrochemical reactions can be performed continuously
during the operation of the electrolytic cell as customary
in the art of electrochemical disinfection or pollution
removal, or using batch process as customary in art of
conventional electrowinning of metals. Both modes of
operation are in accordance with the present invention.
CA 02885437 2015-03-20
- 20 -
[0037] The present invention has been described with
references to the exemplary embodiments arranged for
different applications. While specific values,
relationships, materials and components have been set forth
for purposes of describing concepts of the invention, it
will be appreciated by persons skilled in the art that
numerous variations and/or modifications may be made to the
invention as shown in the specific embodiments without
departing from the spirit or scope of the basic concepts
and operating principles of the invention as broadly
described. It should be recognized that, in the light of
the above teachings, those skilled in the art can modify
those specifics without departing from the invention taught
herein. Having now fully set forth the preferred
embodiments and certain modifications of the concept
underlying the present invention, various other embodiments
as well as certain variations and modifications of the
embodiments herein shown and described will obviously occur
to those skilled in the art upon becoming familiar with
such underlying concept. It is intended to include all such
modifications, alternatives and other embodiments insofar
as they come within the scope of the appended claims or
equivalents thereof. It should be understood, therefore,
that the invention may be practiced otherwise than as
specifically set forth herein. Consequently, the present
CA 02885437 2015-03-20
- 21 -
embodiments are to be considered in all respects as
illustrative and not restrictive.