Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02893310 2015-06-01
;
PROCESS FOR METALS LEACHING AND RECOVERY
FROM RADIOACTIVE WASTES
FIELD OF THE INVENTION
The present invention generally relates to radioactive wastes and more
particularly to a method
of leaching and recovery. More specifically, this invention relates to a
process for metals
leaching and recovery from solid radioactive wastes.
BACKGROUND OF THE INVENTION
In Canada, radioactive wastes are produced since the early '30s, when the
first uranium mine
began operating at Port Radium in the Northwest Territories. Canada is now the
first world
producer of uranium (26% of world production), 90% of its production is
exported and today
there are 20 mines and facilities closed or decommissioned (14 in Ontario, 4
in Saskatchewan
and 2 in the Northwest Territories). Radioactive wastes are grouped into three
categories: nuclear
waste, low and medium activity radioactive waste and waste from the extraction
and
concentration of uranium and rare earths (Low-Level Radioactive Waste
Management Office,
2012). The wastes inventory at the end of 2010 reached 214 million tons from
uranium
processing and 174 million tonnes of mine wastes. Radioactive wastes generated
by uranium
processing and mine wastes (uranium and rare earth) require very specific
decontamination
processes. Atomic Energy of Canada Limited (AECL) is developing a long-term
disposal
strategy for existing cemented radioactive wastes, which contains significant
amounts of
uranium, mercury, and a large number of minor elements, including rare earths
and fission
products. An earlier study indicated that extracting the uranium would be
advantageous for
decreasing the long-term radioactivity of the waste and, consequently, the
cost of the long-term
disposal process. Consequently, there are safety and economic incentives for
the extraction of
metals before subjecting Solid Radioactive Cemented Wastes (SRCW) to a
stabilization process.
Radioactive elements of uranium and thorium are usually associated with rare
earth deposits. The
separation of uranium and thorium from rare earths is often a big concern in
rare earth industry
in order to manage the radioactive nuclides (Zhu et al., 2015). Conversely,
uranium ores often
contain significant concentration of rare earth. Due to recent increases in
both uranium and rare
earth prices, there is renewed interest in uranium and rare earth mine sites
for developing new
1
CA 02893310 2015-06-01
ore bodies as well as re-processing the historic waste rock piles and tailings
impoundments.
Reprocessing Solid Radioactive Mine Wastes (SRMW) may present significant
financial and
environmental benefits.
The technology for recovering uranium from its most common ores is well
established and a vast
amount of information is available in the technical literature (Merritt, 1971;
Wilkinson, 1962).
Uranium is normally leached from its ores with sulfuric acid, separated from
impurities using
solvent extraction or ion exchange, and precipitated with magnesium or
ammonium hydroxide to
yield a commercial product, known as "yellow cake". Extraction of rare earth
is also well
established. The extractive metallurgy of rare earth from monazite sand,
bastnasite ore, and
phosphate rock of igneous origin was described by Habashi (2013). This
includes mineral
beneficiation, leaching methods, fractional crystallisation, ion exchange,
solvent extraction,
precipitation from solution, and reduction to metals. By contrast, cemented
radioactive wastes
(SRCW) differ significantly from common ores. SRCW have a unique mineralogy, a
high nature,
a relatively low U grade, and a high content of Ca (-35%), Si02 (-20%) and Hg
(-1,500 ppm).
The chemical composition of mining radioactive wastes (SRMW) could also differ
significantly
from ores. Some tailings samples are composed of quartz, illite, gypsum,
pyrite, microcline,
calcite and muscovite. Others are mainly composed of gypsum, quartz, nimite,
albite and illite.
The composition of the solid radioactive wastes poses significant impediments
to the extraction
and recovery of metals using conventional technologies. The high Ca content
will interfere with
both carbonate leaching and sulfuric acid leaching by forming large amounts of
CaCO3 and
CaSO4, respectively. Furthermore, the high silica content of the cemented
radioactive wastes
may lead to the formation of colloidal silica, which is known to create severe
problems in
hydrometallurgical circuits (Queneau and Berthold, 1986). Ion exchange was
considered the best
method to separate the uranium or rare earth in the leach solution from the
impurities and to
produce a purified and concentrated solution suitable for yielding a uranium
or rare earth
products. Most likely, solvent extraction technology cannot be used because of
the high
concentrations of Al, Fe and colloidal silica, which may cause severe phase
separation problems
(Queneau and Berthold, 1986; Ritcey and Wong, 1985). The adsorbed uranium and
rare earth are
usually eluted from the resin with dilute acid or alkaline solutions and
subsequently precipitated.
The presence of sodium chloride in uranium and rare earth sulfuric leachate is
a major problem
2
CA 02893310 2015-06-01
for nuclear and mining industries. Several researches were done to improve
selectivity of resins
for metals especially in sodium chloride media.
There is a wide variety of disadvantages and challenges related to the known
techniques for
treating solid radioactive wastes and metals recovery from radioactive
cemented and mine
wastes. Main disadvantages are process efficiency and cost-effectiveness.
There is indeed a need
for a technology that overcomes at least some of the disadvantages of the
known methods in the
field.
SUMMARY OF THE INVENTION
The present invention responds to the above need by providing a process for
metals leaching and
recovery from solid radioactive wastes. Accordingly, the invention provides a
process for
remediation of radioactive wastes comprising metals, which include uranium
and/or cesium
and/or mercury and/or thorium and/or rare earth, and for recovery of these
from radioactive
wastes.
Certain exemplary embodiments provide a process for recovering metals from
solid radioactive
waste comprising the metals, the process comprising:
a) a leaching step comprising contacting the solid radioactive waste with an
aqueous
inorganic acid at a concentration between about 0.1 M and about 2 M, and a
leaching salt, at
a temperature lower than about 100 C, to solubilize at least a portion of the
metals present in
the solid radioactive waste, thereby producing a mixture of a metal-rich
leachate and a metal-
poor waste; and
b) a separation step comprising separating the metal-rich leachate from the
metal-poor waste.
Other exemplary embodiments provide a process for recovering metals from solid
radioactive
waste comprising the metals, wherein the metals comprise uranium, cesium,
mercury, thorium,
rare earth or combination thereof; the process comprising:
a) an attrition step comprising mixing the solid radioactive waste with water
to solubilize at
least a portion of the metals present in the waste providing an aqueous
mixture; and
3
CA 02893310 2015-06-01
separating the aqueous mixture to provide a metal-rich liquid, a metal-
depleted waste and a
metal-rich sludge;
b) a leaching step comprising contacting the metal-rich sludge from step a)
with an aqueous
inorganic acid at a concentration between about 0.1 M and about 2 M, and a
leaching salt, at
a temperature lower than about 100 C, to solubilize at least a portion of the
metals present in
the solid radioactive waste, thereby producing a mixture of a metal-rich
leachate and a metal-
poor waste; and separating the metal-rich leachate and metal-poor waste;
c) a washing step comprising contacting the metal-poor waste from step b) with
an aqueous
solution, at a temperature lower than about 100 C, solubilize a substantial
amount of the
metals to produce a metal-rich solution and metal-poor waste, and separating
the metal-rich
solution and metal-poor waste;
d) a combination step comprising combining the metal-rich liquid of step a),
the metal-rich
leachate of step b) and the metal-rich solution of step c) to provide a metal-
rich combined
solution;
e) a recovery step comprising contacting the metal-rich combined solution with
an ion
exchange resin favoring the metals extraction; or contacting the metal-rich
combined solution
with a coagulant at a pH favoring precipitation of the metals.
Additional embodiments, aspects and features of the invention will be
described and defined
herein.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig 1 is a diagram showing the Cs, Hg, and U solubilisation yields from SRCW
using sulfuric
acid and potassium iodide.
Fig 2 is a diagram showing the Cs, Hg, and U solubilisation yields from SRCW
at different
iodide concentration.
Fig 3 is a diagram showing the Cs, Hg, and U solubilisation yields from SRCW
at different
cement particle size.
4
CA 02893310 2015-06-01
Fig 4 is a diagram showing the Hg recovery from metals-rich solution of
sulfuric acid and
potassium iodide.
Fig 5 is a diagram showing the Cs recovery from metals-rich solution of
sulfuric acid and
potassium iodide.
Fig 6 is a diagram showing the U recovery from metals-rich solution of
sulfuric acid and
potassium iodide
Fig 7 is a diagram showing the U recovery from metals-rich solution at
different iodide
concentration.
Fig 8 is a diagram showing the U elution using sodium hydroxide or a mix of
sodium carbonate
and ammonium nitrate.
Fig 9 is an optical microscope photograph and EDS spectrum of the uranium
precipitate.
Fig 10 is a diagram showing the Cs, lig, and U solubilisation yields with
counter current
recirculation of washing solution
Fig 11 is a diagram showing the U, Th and rare earth solubilisation yields
from SRMW at low
reagent concentration
Fig 12 is a diagram showing the U, Th and rare earth solubilisation yields
from SRMW at high
reagent concentration
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Process embodiments of the present invention provide an effective and
economical technique to
remove metals from solid radioactive wastes and to treat the resulting
leachate solutions. In one
optional aspect of the process embodiments of the present invention, they are
used in relation to
wastes containing uranium, cesium, mercury, thorium, and rare earth.
CA 02893310 2015-06-01
Definitions
"About", when qualifying the value of a variable or property - such as
concentration,
temperature, pH, particle size and so on - means that such variable or
property can vary within a
certain range depending on the margin of error of the method or apparatus used
to evaluate such
variable or property. For instance, the margin of error for temperature may
range between 1 C
to 5 C.
"Radioactive waste" means a radioactive waste that may be in any state,
liquid, solid, granular,
or powder form and so on. It should be understood that the radioactive waste
might be mixed
with a non-radioactive waste at various point in the process in order to form
an overall waste
quantity to meet certain governmental or environmental standards.
"Radioactive cemented waste" means a radioactive waste, which has at some time
been in
contact with a cement to thereby become "cemented". "Radioactive mine waste"
means a
radioactive waste, which was produced during mining operations or related to
mining activities.
It should be understood that the radioactive cemented or mine waste might be
mixed with a non-
radioactive waste at various point in the process in order to form an overall
waste to meet certain
governmental or environmental standards.
"Metals" means the elements of interest which are included in the radioactive
wastes and for
which there are safety and economic incentives for their extraction. Metals
may include uranium,
cesium, mercury, rare earth, a combination thereof and/or other metal species.
"Inorganic acid" means an acid lacking a carbon atom and may be a sulfuric
acid nitric acid,
hydrochloric acid, mixtures thereof, or a combination of acids and
corresponding salts. It should
also be understood that the inorganic acid may be a used or recycled acid.
"Attrition", when pertaining to the solid waste and water, means subjecting a
mixture of solid
waste and water to agitation to induce physical wear of the waste and
separation into smaller
waste particles. Attrition may also aid in desorbing fine waste particles from
larger waste
particles. The attrition may help enable diffusion of the metals from the
waste fractions and/or
fine waste particles into the aqueous solution. The attrition may include
techniques such as
milling. An attrition step may be performed in conjunction with other actions,
such as contacting
6
CA 02893310 2015-06-01
the waste with water, and the contacting step may include soaking, batch
mixing, trickling,
spraying, continuous flow-by, or various combinations of such contacting
techniques.
"Contacting", when pertaining to the solid radioactive waste and the aqueous
inorganic acid,
means that those elements contact each other so as to enable diffusion of the
metals from the
waste phase into the acid solution phase. The "contacting" will often be
referred to as leaching
herein and may include techniques such as soaking, batch mixing, trickling,
spraying, continuous
flow-by, or various combination of such contacting techniques.
"Separating", when pertaining to the metals-rich solution and the metals-poor
waste, means any
suitable solid-liquid separation technique.
"Uranium" (U), "cesium" (Cs), "mercury" (Hg), "thorium" (Th), unless specified
otherwise,
each means a compound containing the given element and may include solubilized
ions,
complexes, derivatives, isomers, as the case may be. For instance, the term
"uranium" may
include uranium (IV) and uranium (VI); "cesium" may include cesium in
association with other
elements or solubilized in an aqueous medium; while "mercury" may include the
element in
association with sulfur or oxygen, solubilized, or in its pure metallic form
upon recovery. Thus,
these elements should be read with a mind to their relationship with the
process steps, process
conditions and other interacting compounds.
"Rare Earth" (REE) means a compound containing at least one element of the
rare earth
elements (Scandium, Yttrium, Lanthanum, Cerium, Praseodymium, Neodymium,
Promethium,
Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium,
Ytterbium, Lutetium) and may include solubilized ions, complexes, derivatives,
isomers, as the
case may be. For instance, the term "Rare Earth" may include one of the light
rare earth (La203
Ce02 Pr60i 1 Nd203) and/or one of the heavy rare earth (Sm203 Eu203 Gd203
Tb407 Dy203
Ho203 Er203 Tm203 Yb203 Lu203 Y203) in association with other elements or
solubilized in an
aqueous medium. Thus, these elements should be read with a mind to their
relationship with the
process steps, process conditions and other interacting compounds.
"Metals-rich solution" means a solution containing the metals removed from the
solid
radioactive waste during a leaching step. It should also be understood that
for subsequent
7
CA 02893310 2015-06-01
treatment of the solution to remove or recover metals, the metals-rich
solution from the initial
step may be combined with solutions from other leaching or washing steps to
form an overall
metals-rich solution. Thus, the metals-rich solution may be combined with
other streams, or be
subjected to various other steps before it is treated to recover one or more
of the metals.
Embodiments of the process
A process for recovering metals from solid radioactive wastes, including
cemented radioactive
wastes and mine radioactive wastes, has been developed. In one aspect, the
process includes at
least one leaching step of the solid radioactive waste to solubilize metals.
Preferred metals can
include uranium, cesium, mercury, thorium and rare earths, or any combination
thereof.
Optionally, the solid radioactive waste can be crushed or screened or any know
methods to
reduce the particle size of the waste. For example, it can be reduce to a
particle size inferior to
about 1 cm, preferably inferior to about 2 mm.
The at least one leaching step is performed with an aqueous inorganic acid
solution, and may be
performed with an additional leaching salt. The aqueous inorganic acid is
preferably in a
concentration from about 0.1 M to about 2 M. The inorganic acid may be
sulfuric acid, nitric
acid, hydrochloric acid, phosphoric acid, or any mixtures thereof, or may also
be a combination
or inorganic acid and corresponding salts. The inorganic acid may be used acid
or recycled acid.
Preferably, the inorganic acid is sulfuric acid.
The leaching salt may be a halogen salt, such as iodine, chlorine, fluorine or
bromine, or any
combination thereof. Preferably, the leaching salt is an iodine salt, such as
potassium iodide,
sodium iodide or the like, or any combination thereof. Most preferably, the
leaching salt is
potassium iodide. The salt may be in a concentration between about 0.012 M and
about 1.2 M.
The solid radioactive waste to be treated by the process may have a content
from about 50 g/L to
about 500 g/L of the total mixture of the leaching step.
The at least one leaching step is preferably carried out at a temperature
lower than about 100 C
to solubilize at least a portion of the metals present in the solid
radioactive wastes, preferably
between about 20 C and about 80 C. Optionally, the leaching step may further
comprise any
form of mixing to aid the solubilization. Mixing may be carried out for a
period sufficient to
8
CA 02893310 2015-06-01
solubilize the metals, for example from about 0.5 h to about 24 h. A metal-
rich leachate and a
metal-poor waste are formed from the leaching step.
In another embodiment, multiple leaching steps may be carried out, for example
in a sequential
manner. Each leaching step may independently comprises the same or different
conditions,
namely the inorganic acid and its concentration, the leaching salt and its
concentration, the
temperature, the optional mixing and mixing time. The multiple leaching steps
may be
performed in batch, semi-continuously or continuously in tank reactors, or any
known methods
in the art.
In another aspect, the process further includes a separation step for
separating the metal-rich
leachate and a metal-poor waste. For example, the separation step may comprise
decantation,
filtration, centrifugation, solid-liquid separation or any suitable known
techniques, or any
combination thereof.
The present process may further comprise at least one treatment step for the
recovery of metals
from the metal-rich leachate. This recovery step may be carried out using any
known methods in
the art for recovering the desired metal, preferably uranium, cesium, mercury,
thorium, rare
earths, any combination thereof, and may be recovered in the form of a mixed
metalloid or as a
pure metal. For example, the recovery step may comprise chemical
precipitation, ion exchange,
solvent extraction or adsorption, or any combination thereof. Preferably, the
metals are recovery
by ion exchange or by a coagulant favoring the precipitation of the metals at
a given pH.
Preferably, at least two metals are recovered from the metal-rich leachate,
most preferably all
metals are simultaneously recovered. It has been shown that the removal yields
may be
increased when using the leaching salt rather than when only the inorganic
acid is used, as will
be exemplified in the below examples, tables and figures.
In a further embodiment, the metal-poor waste obtained from the separation
step may be washed
to potentially remove any residual metals, by at least one washing step,
optionally multiple
washing steps. This washing step may comprise filtering the separated metal-
poor waste to
provide solids, rinsing or mixing the solids with a washing solution and
separating the washed
solids and the spent washing waters. The washing solution may comprise water,
a dilute acid
solution, or an acid solution. When proceeding with multiple washing steps,
the conditions as
9
CA 02893310 2015-06-01
previously defined may be independently selected. The spent washing waters may
contain
residual metals and may be combined with any previous fraction or treated
directly by any steps
as defined above for recovering the metals. For example, it may be recycled
into a further
leaching step or directly undergo a recovery step as previously defined.
In another aspect, the process may include, before the at least one leaching
step, at least one
attrition step where the solid waste is mixed with water and subjected to
attrition so as to
solubilize metals from the solid waste into water.
According to one embodiment of the process, the attrition step includes mixing
the waste with
water. The attrition step further includes agitating the aqueous mixture of
waste for a period
sufficient to adequately solubilize metals present in the waste and form an
aqueous suspension of
waste including waste fractions and fine waste particles. Optionally, the
agitation duration may
be between about 0.01 h and about 1 h. The attrition step may therefore be
performed to
solubilise in water at least a portion of metals present in the initial solid
waste.
Optionally, the amount of water and/or the amount of solid waste that are
mixed together during
the attrition step may be adjusted to obtain an aqueous mixture of waste
having a waste
concentration between about 50 g/L and about 500 g/L of solution.
According to another embodiment of the process, at least one attrition step
may be a single
attrition step or include several sequential attrition steps. Optionally, the
attrition step(s) may be
operated in batch, semi-continuous or continuous mode in tank reactors.
In another aspect, prior to the at least one attrition step, the process may
include crushing or
screening of the solid waste as defined above.
In another aspect, subsequent to the at least one attrition step and before
the at least one leaching
step, the process may include a first separation step to separate the aqueous
suspension of waste
into a metal-rich liquid, a metal-rich sludge (also referred to as attrition
sludge) and a metal-
depleted waste.
Optionally, the separation step may include decantation, filtration,
centrifugation, or another standard technique of solid-liquid separation known
in the art.
CA 02893310 2015-06-01
It should be understood that the waste which is subjected to the leaching step
may be a solid
waste as defined above, the metal-depleted waste as defined above if the
process includes an
attrition step prior to the leaching step, or a combination thereof.
In another aspect, the process may further include combining the metal-rich
liquid from the
attrition step, the metal-rich leachate from the leaching step and the spent
washing liquids from
the washing step to obtain a solution containing a major portion, or close to
the totality of the
targeted metals. Optionally, some or all of the washing liquids may also be
directly used as
process water for the operation of the initial leaching step for a subsequent
batch of solid waste
to be treated according to the present process.
In another aspect, the process may also include treating the metal-rich
solution, the metal-rich
acid leachate, the spent washing liquids or a combination thereof, to recover
at least one of the
metals. The combination of the metal-rich solution, the metal-rich acid
leachate and the spent
washing liquids will be generally referred to herein as the "metal solution",
which contains the
solubilized metals. It should be understood however that the solution treated
to recover
solubilized metals may be the metal-rich solution or the acid leachate or the
spent washing liquid
only.
Optionally, the recovery step may include one or a combination of known
techniques as defined
above. After the metal solution has been treated to remove the metals, it may
for example be
used as process water for the operation of the leaching step. Optionally, the
leaching residue and
the metals extracted from the waste can be safely disposed or recycled.
In one aspect, a process for recovering metals from solid radioactive waste is
provided,
comprising an attrition step as defined above, a leaching step as defined
above, a washing step as
defined above, a combination step comprising combining the metal-rich liquid
from the attrition
step, the metal-rich leachate from the leaching step and the metal-rich
solution from the washing
step, and further comprises a recovery step as previously defined to recover
at least one of
uranium, cesium, mercury, thorium or rare earths.
Embodiments of the present invention provide a number of advantages.
Advantages will be
understood as per the above and the examples and experimental data obtained
through the
11
CA 02893310 2015-06-01
"
extensive studies presented below. For instance, the use of inorganic
acid and leaching salt, such
as sulfuric acid and potassium iodide respectively allows very efficient
uranium, cesium,
mercury, thorium, and/or rare earth solubilisation from waste at a low
chemical cost.
Furthermore, the addition of at least one washing step after the leaching step
is useful to remove
the dissolved metals still present in the waste. Selective separation and
purification of metals by
ion exchange allows to safely disposing or recycling the metals. Finally,
extracting the metals
would be advantageous for decreasing the radioactivity of solid radioactive
waste and,
consequently, the cost of long-term disposal.
EXAMPLES, EXPERIMENTATION & ADDITIONAL INFORMATION
The embodiments of the present invention will be further comprehended and
elaborated in light
of the following examples and results, which are to be understood as exemplary
and non-limiting
to what has actually been invented.
General Methodology
The following describes the general methodology of examples of an embodiment
of the process
of the present invention.
Radioactive cemented wastes
All the experiments were carried out with solid radioactive cemented wastes
(SRCW) prepared
at CanmetMINING. The procedure involves mixing a synthetic solution with
either General Use
(GU) or High Early Strength (HE) cements manufactured by Lafarge Canada Inc.,
in a manner
that mimics the process carried out at AECL. The solid wastes batches were
allowed to age and
were subsequently removed from the pails with an air hammer. The whole pail
content (-20 kg)
was crushed in a laboratory jaw crusher (Retsch, model BB200) to about 2 mm
and then split
into 12 fractions of about 1.7 kg each, using a large capacity (20 kg) rotary
splitter (GENEQ,
model SE040J-001). One of the 12 fractions was further ground in a disc mill
(Retsch, model
DM200) to less than 300 ptm and split into ten fractions of about 170 g, using
a medium capacity
rotary splitter (Fritsch Rotary Cone Sample Divider, Model Laborette 27), then
re-sampled at
either 100 g or 50 g.
12
CA 02893310 2015-06-01
Table 1 shows the experimental conditions used to prepare various SRCW batches
and their
partial compositions.
Table 1: Main experimental parameters used to prepare solid wastes batches
and partial composition
Batch Cement SIC* Aging Aging
Cs ppm Hg ppm U ppm
Code Type Ratio Time Temperature
U13 GU 0.29 18 months 60 C 3.5 1,116 893
U28 GU 0.39 18 months 60 C 8.5 1,973
1,150
U29 GU 0.29 18 months Ambient 7.2 1,977
1,104
U32 GU 0.29 18 months 60 C 6.0 1,277 850
U34 HE 0.39 18 months Ambient 8.0 2,651
1,355
*Solution to Cement ratio
Radioactive mine wastes
Experiments on solid radioactive mine wastes (SRMW) were conducted using
submerged
tailings collected from the two uranium mines and using an ore concentrate of
rare earth. The
rare earth ore concentrate was used as received. A field campaign was
conducted in June 2012 at
the Denison mine (Elliot Lake, Ontario). Various cores and four bulk samples
were taken at the
Denison tailings management area. The following summer, other samples were
taken at the
Gunnar mine site (Saskatchewan) at the Langley Bay. Once received, the samples
were stored at
4 C and remained saturated with water to prevent oxidation.
Table 2 shows the elements concentration in submerged tailings from uranium
mines and the ore
concentrate of rare earth. Mineralogy of these samples indicated that Quest
and Denison
samples are mainly made of Quartz, K-feldspar and pyroxene minerals. Gypsum
phases are
mainly present in the Gunnar sample.
13
CA 02893310 2015-06-01
Table 2: Elements concentration in tailings from uranium mines and the ore
concentrate of rare
earth
Elements
Th U Y La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
(mg/kg)
Quest 585 109 4816 1383 3427 388 1262 340 22 397 100 743 182 615 90 537 74
Denison 213 65 32 472 854 85 272 42 2 31 3 9 1 2 0 2 0
Gunnar 39 40 32 86 165 18 59 10 1 9 1 6 1 3 0 3 0
Leaching experiments
The screening leaching tests were done by mixing 50 g of SRCW or SRMW with a
known
volume of a selected lixiviant in a 1-L glass Erlenmeyer flask, which was
agitated in a
temperature-controlled orbital shaker (Labnet, Model 211DS). The optimization
and validation
leaching tests were performed in a 2-L beaker. Leaching steps were done by
mixing 50 g of
waste with 500 mL of solution for a pulp density (PD) of 10% v/w. Agitation
was performed
using an immersed axial impeller or by magnetic stirring set at 300 rpm. The
solution was then
filtered onto WhatmanTM No. 4 cellulose paper (porosity = 20-25 p.m). The
residue and filter
were then dried at room temperature.
Metals recovery
Experiments regarding ion exchange resin and adsorbent assessed the potential
of ion exchange
for selective recovery of metals. Experiments were done in batch and
continuous mode (on
column). One gram of resin was mixed with 100 mL of leachate in 500 mL
Erlenmeyer flasks
and shake at 200 revolutions per minute (rpm) (Orbital shaker, Lab-line
Environ-Shaker, model
3528) for 24 h to ensure that chemical equilibrium was attained. Thereafter,
liquid to solid
separation was made by filtration onto Whatman 934AH filter. Cesium and
mercury were
removed selectively by using potassium cobalt hexacyanoferrate (KCFC) and
resin LewatitTM
TP214 respectively. For uranium, the ion exchange resins studied were
ReillexTM HPQ,
DOWeXTM 21K-XLT, Lewatit MP500, Lewatit TP207, Dowex M4195, Lewatit TP260, and
Lewatit K7367. The column experiments were done using an OmnifitTM column with
a bed
volume of 12 mL. Leachate was passed through the resin using a peristaltic
pump
14
CA 02893310 2015-06-01
(MasterflexTm) at a flow rate of 3 BV/h. An automatic fraction collector
(Eldex Laboratories)
was used to take samples of the column effluent. Uranium was then eluted form
the resin with
1M Na2CO3, 1M NH4NO3, 6M NaOH, or 2M HNO3 solutions and subsequently
precipitated
using sodium hydroxide, hydrogen peroxide solution (30%), ammonium hydroxide
solution
(28%) or magnesium hydroxide.
Chemical precipitation
Experiments occurred in 250 mL beaker with magnetic stirring at 250 rpm using
a TeflonTm-
covered bar. Solution pH was initially stabilized to the appropriate pH by
adding alkaline or acid
solution. The supernatant was collected and filtrated on Whatman 934AH
membranes for further
soluble metals analysis. Uranium precipitation from the ion exchange resin
eluate was done
using ammonium hydroxide solution (28%), magnesium or sodium hydroxide.
Analytical
The liquid samples were analyzed by ICP-MS (Thermo-Fisher Scientific, X-Series
II), after
appropriate dilution with HC1 to stabilize the Hg(II). Solid samples were
digested in HC1 before
being analyzed by ICP-MS. The mineralogical characterization of the cemented
material and
leach residues was done using a combination of X-Ray Diffraction (XRD)
(Rigaku, model
D/Max), Scanning Electron Microscopy (SEM) (JEOL, model JSM 820) and Variable-
Pressure
Scanning Electron Microscopy (VP-SEM) (Hitachi, model S-3200N) both with
Energy
Dispersive X-Ray Analyzer (EDS).
EXAMPLE 1: URANIUM, MERCURY, AND CESIUM LEACHING FROM SRCW
Without being bound by theory, the concentrated sulphuric acid can act both as
an acid and as an
oxidising agent. The concentrated sulphuric acid gives a hydrogen ion to the
halide ion to
produce a hydrogen halide. As an example, concentrated sulphuric acid reacts
with sodium
chloride to produce hydrogen chloride and sodium hydrogensulphate. All of the
halide ions
(fluoride, chloride, bromide and iodide) behave similarly. Fluoride and
chloride ions will not
reduce concentrated sulphuric acid. Iodide ions are stronger reducing agents
and are oxidised to
iodine by the concentrated sulphuric acid. Sodium chloride formed mercury
complex (HgC142-,
Kf = 5.1015) when Hg is present as mercury oxide Hg0 or metallic mercury Hg
in 30 months
CA 02893310 2015-06-01
aged SRCW but not with mercury sulfide HgS (pK = 52) formed in 60 C cured
SRCW. In order
to oxidize Hg , as well as HgS, tests were performed using iodide halogen salt
as strong oxidant
to form Hg complex (HgI42", Kf = 2.103 ). As background, it can be mentioned
that a patented
process was developed (Fousts, 1993) for soil remediation by removing mercury
using a
treatment with an oxidant, such as iodide, and a complexing or solubilising
agent, such as
potassium iodide. In addition, Klasson et Koran (1997) studied the removal of
Hg from solids
using a potassium iodide/iodine leaching process.
Various SRCW cured at 60 C and/or aged during 30 months were subjected to
leaching using
sulfuric acid and potassium iodide. These SRCW were U13 (18 months at 60 C),
U28 (30
months at 60 C), U29 (30 months at ambient temperature), U32 (30 months at 60
C), and U34
(30 months ambient temperature). A 50 g sample of SRCW crushed at 0.3 mm was
mixed with
500 mL of distilled water to obtain a 10% pulp density. Potassium iodide was
also added to
obtain a concentration of about 1.2M. Then pure sulfuric acid was added to
obtain a
concentration of about 1M. Agitation using an immersed impeller during 2 hours
at ambient
temperature was performed.
Figure 1 presents the solubilisation of Cs, Hg, and U from five mentioned SRCW
using sulfuric
acid and potassium iodide: U13 (18 months at 60 C), U28 (30 months at 60 C),
U29 (30 months
at ambient temperature), U32 (30 months at 60 C), and U34 (30 months ambient
temperature)
(particle size = 0.3 mm, t = 120 min, H2SO4 = 1M, PD = 10%, T 20 C, KI =
1.2M). Initial
concentration of Cs, Hg, and U in each SRCW are given in Table 1. For all the
tested SRCW
batches, solubilisation yields are above 97% for Cs and 98% for U and Hg.
Sulfuric acid and
potassium iodide improve the solubilisation of Hg by oxidation of all the
mercury species and
form mercury tetraiodide complex (HgI42-, Kf = 2.1030).
EXAMPLE 2: INFLUENCE OF IODIDE CONCENTRATION AND PARTICLE SIZE
Optimization tests were done to evaluate the influence of potassium iodide
concentration and
SRCW particle size on the solubilisation efficiencies. The optimization
experiments were done
in a 2-L beaker by mixing 50 g of U29 SRCW with 500 mL of solution for a pulp
density of 10%
v/w. Leaching of U29 cemented waste was performed using different
concentration of KI from
0.01M to 1.2M. Figure 2 and 3 present the influence of the iodide
concentration on Cs, Hg, and
16
CA 02893310 2015-06-01
U solubilisation. In Figure 2, Cs, Hg, and U solubilisation yields from U29
cemented waste are
shown, using sulfuric acid and various concentrations of potassium iodide.
Initial concentrations
are about 7.2 ppm for Cs, 1 977 ppm for Hg, and 1 104 ppm for U (particle size
= 0.3 mm, t =
120 min, H2SO4 = 1M, PD = 10%, T = 20 C). Figure 2 shows no decrease of
solubilisation
yields for potassium iodide concentration from 1.2M to 0.2M. At 0.1 M and
lower concentration
of KI, the process efficiency decreases slightly especially for Hg. This
result indicates the
importance of potassium iodide to oxidize the mercury species in the SRCW.
Potassium iodide is
effective even at low concentrations of 0.1M, whereas a high concentration of
sodium chloride of
4M is needed to achieve good Hg solubilisation.
Another set of experiments were done using a particle size from 0.3 mm to 6
mm. Figure 3
presents the influence the cement particle size on Cs, Hg, and U
solubilisation. Cs, Hg, and U
solubilisation yields from U29 cemented waste are shown, using sulfuric acid
and potassium
iodide. Initial concentrations are about 7.2 ppm for Cs, 1 977 ppm for Hg, and
1 104 ppm for U
(t = 120 min, H2SO4 = 1M, PD = 10%, T = 20 C, KI = 1.2M). Figure 3 shows that
the particle
size of the cemented waste has an important influence on the solubilisation
efficiency. The
increase of the particle size decreases the solubilisation efficiency. For
example, mercury
solubilisation decreases form 99% to 95% and then to 90% when particle size
increases from 0.3
mm to 2 mm and then to 4 mm. These results may be explained by the mineralogy
of the
cemented waste. Uranium phases were found mainly as long layers (400 lam) and
some small
grains. Mercury phases were found as grains below 20 vtm and the small grains
that tend to
agglomerate with longer aging times and higher aging temperatures. Moreover,
the proportion
of metallic mercury and mercury sulfide appears to increase with aging times
and aging
temperatures.
EXAMPLE 3: CESIUM AND MERCURY SELECTIVE RECOVERY
Cesium and mercury were removed selectively from the leachate by using
Potassium Cobalt
Hexacyanoferrate (KCFC) and Lewatit TP214 respectively. Column experiments
were done to
further test the selectivity of the Hg and Cs absorbents and evaluate their
capacity. The leachate
with ¨100 ppm of Hg was passed through a 12 mL Omnifit column filled with
Lewatit TP214
resin at 3 bed volumes per hour (BV/h). Figure 4 presents the mercury uptake
by the resin, which
17
CA 02893310 2015-06-01
illustrates the Hg recovery from cemented waste leaching using sulfuric acid
and potassium
iodide.
Mercury was selectively removed from the leachate as no cesium and uranium
were retained by
the resin. Other experiments indicated that Hg(II) was partially adsorbed in
the absence of
chloride or iodide ions and strongly adsorbed in the presence of even trace
amounts of chloride
or iodide ions. A mercury loading in the resin of 5% was achieved and could
reached 50%
according to literature.
Figure 5 shows the cesium uptake by passing the same leachate with ¨0.8 ppm Cs
through a
small glass column (1 mL) filled with KCFC at a 3 BV/h flovvrate. Figure 5
indicates a selective
recovery of cesium from the leachate. For the mercury loading, the
breakthrough was not
achieved due to the high capacity of the ion exchange resin used. Similarly,
the cesium
breakthrough was not achieved due to the high capacity of the KCFC.
After the cesium and mercury recovery, no change in uranium concentration was
observed.
Cesium removal could be done as a final step treatment as cesium does not
interfere with
uranium recovery. However, the Hg loading reduces to some extent the resin
capacity for U.
Therefore, the removal of Hg must be preferably prior to the removal of U.
EXAMPLE 4: URANIUM SELECTIVE RECOVERY
The presence of sodium chloride in sulfuric leachate is a major problem for
nuclear and mining
industries. Several investigations were done to improve the selectivity of
resins especially in
sodium chloride media. In case of U recovery from sulfuric acid leachate
containing potassium
iodide, no studies exist in the literature. Zhang et al. (2012) have
investigated the recovery of
gold from iodine¨iodide solutions using an anion exchange resin. The gold
iodide complex can
be effectively loaded on the resin provided the resin is not heavily loaded
with triiodide. Sodium
chloride solution containing sulfite was found to be highly effective for the
elution of both gold
and iodine (Zhang et al. 2012).
Ion exchange experiments were performed in batch mode and then in column mode
to recover U
from sulfuric leachate in iodide media. Several ion exchange resins were
studied including
strong anionic resins (Dowex 21K XLT, Lewatit K7367 and Lewatit MP500),
chelating resins
18
CA 02893310 2015-06-01
(Dowex M4195, Lewatit TP207 and Lewatit TP260), and weak anionic resin
(Reillex 425).
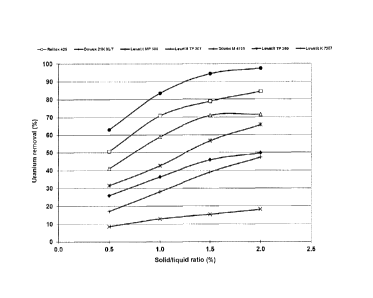
Figure 6 presents the U recovery by theses resins in batch mode. For this
purpose, mass of 0.5 g
to 2 g of dry resin was added to 100 mL of sulfuric leachate in 50 g/L iodide
media with an
initial U concentration of 40 ppm. Agitation was performed using an orbital
shaker at 100 rpm
during 24 h to ensure absorption equilibrium is reached. Figure 6 is a
comparison of ion
exchange resin for U recovery from the leaching using sulfuric acid and
potassium iodide and
shows that U recovery from 50 g/L potassium iodide leachate using the
chelating resin Lewatit
TP260 is the most efficient. Uranium recovery yield reached 97% with a solid
liquid ratio of
2%.
The Lewatit TP260 resin was selected to perform several additional ion
exchange tests with
sulfuric acid in potassium iodide media leachates obtained in previous
leaching experiments. In
column experiments were conducted with an Omnifit column with a bed volume of
12 mL filled
with the Lewatit TP260. Leachate was passed through the resin using a
peristaltic pump
(Masterflex) at a flowrate of 3 BV/h (0.6 mL/min) for a retention time of
about 20 min. A
fraction collector was used to split the column effluent in 24 sequential
fractions. Several tests
were done using different iodide concentration from 1 g/L to 100 g/L. Initial
U concentrations
were about 40 ppm for 1, 10, and 50 g/L leachates and 80 ppm for 100 g/L
leachate. The results
are shown in Figure 7. Uranium uptake is represented by the difference between
initial and final
concentration in the leachate (C/C0). In such representations, the lower the
curve is, the better
the U uptake is. Uranium uptake by the resin seems to be improved by the
presence of higher
iodide concentration. Best breakthrough was obtained with the 50 g/L KI
leachate. A good U
uptake was also obtained with the 100 g/L KI leachate, which has the highest
initial
concentration. Resin capacity reached 6 mg/g and breakthrough was obtained at
60 BV.
EXAMPLE 5: URANIUM ELUTION AND PRECIPITATION
Several experiments were done to produce yellow cake from the elution
solutions. Ion exchange
experiments for U recovery from sulfuric leachate with potassium iodide were
conducted using
the ion exchange resin Lewatit TP260. Uranium loaded in the Lewatit TP260
chelating resin
was eluted using several stripping reagents. The experiments were done using
2M HC1, 6M
NaOH and a mix of 1M Na2CO3 and NRINO3 solutions.
19
CA 02893310 2015-06-01
Figure 8 presents the U elution curves obtained from the Omnifit column filled
with Lewatit
TP260 resin at 1.5 BV/h flowrate. The curve using HC1 is not presented, as
uranium was not
significantly eluted from the resin. The mix of sodium carbonate and ammonium
nitrate allows
very good uranium stripping from the resin in 4 BV and produce an uranium-
bearing solution
suitable for further precipitation. The concentration factor obtained by U
loading and stripping is
about 15 (60 BV for loading / 4 BV for stripping). Additional resin in pulp
tests were done to
recover uranium from the Lewatit TP 260 using 2M HNO3, 1M NaC1, 2M NaOH, 1M
Na2CO3 /
N114NO3, 2M NH4OH and 1M Na2CO3 as stripping reagents. The best uranium
stripping from
the resin was obtained using 1M sodium carbonate which allows 99% of uranium
recovery.
Uranium was then recovered as yellow cake using several precipitation reagents
including
ammonium hydroxide (ammonium diuranate), sodium hydroxide (sodium diuranate),
magnesium
oxide (uranium trioxide) and hydrogen peroxide (uranium peroxide). Several
precipitation tests
were done. Uranium was precipitated as sodium diuranate (SDU) using sodium
hydroxide.
Then the crude yellow cake was purified by precipitation using hydrogen
peroxide (uranium
peroxide) or ammonium hydroxide (ammonium diuranate) to obtain pure yellow
cake. Figure 9
presents an optical microscope photograph and the typical EDS spectrum of the
SDU yellow
cake from the U recovery in sulfuric and iodide media and precipitation using
sodium hydroxide.
The EDS spectra of the yellow cake indicates it is a Na-U-0 compound with a
high
concentration of uranium and minor impurities of Si, P, and Al. The XRD
pattern indicates the
yellow cake is partially crystalline and identifies it as mainly a sodium
uranium oxide.
EXAMPLE 6: REUSE OF WASHING SOLUTIONS FOR SUBSEQUENT LEACHING
The U29 SRCW was subjected to several leaching using sulfuric acid and
potassium iodide. The
leaching steps were carried out by using washing solution instead of water.
After the leaching
steps, washing steps were done by using water. The leaching and washing steps
were performed
using a counter current recirculation of washing solution. The reuse of
washing solution allows
reducing the process costs and the volume of liquid waste produce. Five 150 g
sample of SRCW
crushed at 2 mm were mixed with 1500 mL of washing solution to obtain a 10%
pulp density.
Potassium iodide was also added to obtain a concentration of about 0.06M. Then
pure sulfuric
acid was added to obtain a concentration of about 1M. Agitation using an
immersed impeller
CA 02893310 2015-06-01
during 2 hours at ambient temperature was performed. After a filtration step,
the leaching
solution were treated to remove cesium and mercury, and then to recover the
uranium. The
treated-leaching solution is recycled and reused for the washing steps. Figure
10 presents the
solubilisation of Cs, Hg, and U from SRCW using sulfuric acid and potassium
iodide (particle
size = 2 mm, t = 120 min, H2SO4 = 1M, PD = 10%, T = 20 C, KI = 0.06M). Initial
concentration of Cs, Hg, and U of U29 SRCW are given in Table 1.
According to Figure 10, solubilisation efficiencies increase for each elements
during the five
recirculation experiments. Improvement of solubilisation is due to the
recycling of chemical
reagents which concentration increase during counter-current recirculation.
These results show
that the washing solution can be reused in this process without affecting the
leaching
performance. The leaching solution can also be reused for washing steps after
the recovery of
metals by the separation step.
EXAMPLE 7: RARE EARTH LEACHING FROM SOLID RADIOACTIVE MINE
WASTES
Experiments on solid radioactive mine wastes (SRMW) were conducted using
submerged
tailings collected from the two uranium mines (Denison and Gunnar) and using
an ore
concentrate of rare earth (Quest). Table 2 shows the elements concentration in
submerged
tailings from uranium mines and the ore concentrate of rare earth. Theses SRMW
were
subjected to leaching using sulfuric acid and potassium iodide. A 100 g sample
of SRMW was
mixed with 1000 mL of distilled water to obtain a 10% pulp density. Agitation
using an
immersed impeller during 2 hours at ambient temperature was performed. Initial
concentration of
U, Th, and rare earth in each SRMW are given in Table 2.
In the first set of experiments, metals solubilisation from SRMW (Quest ore
concentrate,
Denison and Gunnar tailings) were done by using sulfuric acid and potassium
iodide at low
reagent concentration. Potassium iodide was added to obtain a concentration of
about 0.06M.
Then pure sulfuric acid was added to obtain a concentration of about 1M.
Figure 11 presents the
U, Th, and rare earth solubilisation yields from SRMW (Quest ore concentrate,
Denison and
Gunnar tailings) using sulfuric acid and potassium iodide at low reagent
concentration (t = 120
min, H2SO4 = 1M, PD = 10%, T = 20 C, KI = 0.06M).
21
CA 02893310 2015-06-01
In the second set of experiments, metals solubilisation from SRMW were done by
using sulfuric
acid and potassium iodide at high reagent concentration. Potassium iodide was
added to obtain a
concentration of about 0.6M. Then pure sulfuric acid was added to obtain a
concentration of
about 2M. Results of U, Th, and rare earth solubilisation yields from SRMW (t
= 120 min,
H2SO4 = 2M, PD = 10%, T = 20 C, KI = 0.6M) are presented in Figure 12.
For the Quest ore concentrate, solubilisation yields at low reagent
concentration reached 96% for
Th, 59% for U, 20% for light rare earth and 40% for heavy rare earth. At high
reagent
concentration, solubilisation yields of 100% for Th, 93% for U, 41% for light
rare earth and 76%
for heavy rare earth were achieved. For the Denison tailings, solubilisation
yields at low reagent
concentration reached 63% for Th, 33% for U, 3% for light rare earth and 37%
for heavy rare
earth. At high reagent concentration, solubilisation yields of 95% for Th, 52%
for U, 5% for
light rare earth and 61% for heavy rare earth were achieved. For the Gunnar
tailings,
solubilisation yields at low reagent concentration reached 38% for Th, 50% for
U, 43% for light
rare earth and 67% for heavy rare earth. At high reagent concentration,
solubilisation yields of
48% for Th, 51% for U, 51% for light rare earth and 84% for heavy rare earth
were achieved.
The process for metals leaching from solid radioactive wastes using sulfuric
acid and potassium
iodide is also efficient for U, Cs, and Hg recovery from radioactive cemented
wastes as well as
for U, Th, and rare earth recovery from radioactive mine wastes.
22
CA 02893310 2015-06-01
REFERENCES
Foust D. F. 1993. Extraction of mercury and mercury compounds from
contaminated material
and solutions. US Patent 5,226,545.
Habashi F., 2013. Extractive metallurgy of rare earths. Canadian Metallurical
Quaterly, 52, 3.
Klasson T. K., Koran L. J. 1997. Removal of mercury from solids using the
potassium
iodide/iodine leaching process. Oak Ridge National Laboratory. Oak Ridge
Tennessee
Kraus K.A. and Nelson F., 1956. "Anion exchange studies of the fission
products". Proceedings
of the First International Conference on the Peaceful Uses of Atomic Energy.
Columbia
University Press, New York, NY, USA. Vol. 7, pp 113-125.
Merritt R.C., 1971. The extractive metallurgy of uranium. Colorado School of
Mines Research
Institute. Johnson Publishing Company, Boulder, Co., U.S.A.
Obermoller H.R., White D.A. and Lagos S., 1991. "Resin adsorption of anionic
chloride
complexes for uranium isotope chemical exchange reactions". Hydrometallurgy,
27, 63 74.
Queneau P.B. and Berthold C.E., 1986. "Silica in hydrometallurgy: An
overview". Can. Met. Q.,
25(3), 201-209.
Ritcey G.M. and Wong E.W., 1985. "Influence of cations on crud formation in
uranium circuits".
Hydrometallurgy, 15(1), 55-61.
Wilkinson W.D., 1962. "Uranium Metallurgy, Volume I (Uranium Process
Metallurgy)". John
Wiley and Sons.
Zhang H., Jeffery C.A., Jeffrey M.I. 2012. Ion exchange recovery of gold from
iodine¨iodide
solutions. Hydrometallurgy 125-126 (2012) 69-75.
Zhu Z., Pranolo Y., and Cheng C.Y. 2015. Separation of uranium and thorium
from rare earth for
rare earth production ¨ A review. Minerals Engineering 77, 185-196.
23