Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
METHOD OF DIRECT REDUCTION OF CHROMITE WITH CRYOLITE ADDITIVE
TECHNICAL FIELD
[0001] The present invention relates to chromite reduction.
BACKGROUND
[0002] Chromium (Cr) is an industrially important element,
necessary for chrome plating and the production of
stainless steel. The only source of metallic chromium
that exists is chromite ore (Cr203), which commonly
occurs as chromite (FeCr200 where iron in the formula
can be substituted by magnesium and chromium by both
aluminum and ferric iron. Ferrochrome smelting using
conventional carbothermic methods is an energy-
intensive process, requiring energy inputs up to 4.6
MWh for each tonne of ferrochrome produced.
[0003] "Prereduction" (direct reduction of the chromite ore
before smelting) can allow reduction and metallization
to occur at lower temperatures, thus requiring less
energy. In this context, "reduction" and
"prereduction" refer to a chemical process wherein
oxygen is removed from one reactant (here, the
chromite ore) and taken up by another reactant
(referred to as the "reductant"). Hence, the
oxidation states of the constituents of one reactant
(the chromite here) is "reduced".
[0004] Although prereduction enables lower temperatures, the
process occurs in solid-state (meaning that both the
chromite ore and the reductant are in solid form).
- 1 -
CA 077613 2020--31
W02019/075545
PCT/CA2017/051252
Solid-state reactions are kinetically slow and rarely
result in completely metallized chromite ore. The
greater the metallization during prereduction, the
lower the energy requirements can be, and the greater
the energy savings.
[0005] Additionally, low ash coke, the most common reductant
source in the smelting process, is expensive in
itself. A method that increases metallization before
smelting without requiring a high quality reductant
would be more cost-effective than traditional smelting
processes.
[0006] It is common to add fluxing agents to the reduction
furnace, to improve the metallization rate. These
fluxing agents enhance the formation of a liquid slag
layer in the chromite ore and allow greater
metallization at lower temperatures. Several kinds of
fluxing agent have been considered in the prior art,
including alkali salts, borates, carbonates and
silicates. Addition of these fluxes decreases the
melting temperature of refractory oxides namely, MgO
and A1203. This has enabled chromite reduction to
occur effectively even at temperatures under 1400 C
(as compared to reduction temperatures of up to 2000 C
for smelting).
[0007] However, not all of these fluxes are easily available.
They may be expensive, uncommon, or both. Thus, there
would be a benefit to the use of an additive that is
not only useful, but also widely available and cost-
effective. Preferably, such an additive would allow
for chromite reduction at even lower temperatures.
- 2 -
Attorney Docket No. 1327P001CA01
SUMMARY
[0008] The present invention provides a method of chromite
reduction using cryolite (Na3A1F6) as an additive. The
additive used may be pure cryolite or an impure
mixture containing cryolite, such as the bath material
produced as waste or as a by-product of aluminum
smelting processes. Unlike regular fluxing agents that
enhance the slag (oxide based liquid phase) forming
process, cryolite unlocks the complex oxide structure
by selectively dissolving various oxides from the
chromite/spinel. Cryolite is known to be a corrosive
salt in molten form that selectively dissolves the
refractory components (MgO and A1203). The molten
cryolite layer acts as a transport medium for Cr and
Fe species.
[0009] In one embodiment, the reduction product is melted at
a higher heat after reduction, to form larger metallic
particles. In another embodiment, the chromite ore is
granulated with cryolite particles and carbon
reductant particles before being reduced.
[0010] The present invention provides a method of reducing
chromite ore comprising the steps of:
(a) reducing a mixture in a furnace to form a reduction
product;
(b) separating said reduction product into a metallic
chromium alloy phase and a non-metallic phase,
wherein said mixture is a mixture of chromite ore
particles, reductant particles, and cryolite additive
particles.
- 3 -
Date Recue/Date Received 2020-07-30
Attorney Docket No. 1327P001CA01
[0010a] In another aspect, this document discloses a method
for direct reduction of chromite, said method
comprising the steps of: (a) reducing a mixture to
form a solid reduction product; (b) separating said
solid reduction product into a metallic chromium alloy
phase and a non-metallic phase, wherein said mixture
comprises of a mixture of chromite particles,
reductant particles, and a transport media, said
transport media being cryolite particles.
[0010b] In another aspect this document discloses A method for
direct reduction of chromite, said method comprising
the steps of: (a) mixing chromite particles, reductant
particles, and a transport media, said transport media
being cryolite particles, to form a mixture; (b)
reducing said mixture to form a solid reduction
product; (c) cooling said solid reduction product; and
(d) separating said solid reduction product into a
metallic chromium alloy phase and a non-metallic
phase.
[0010c] In another aspect, this document discloses a method
for direct reduction of chromite, said method
comprising the steps of: (a) obtaining chromite
particles; (b) obtaining reductant particles; (c)
obtaining cryolite particles; (d) mixing said chromite
particles, said reductant particles, and said cryolite
particles to form a mixture; (e) reducing said mixture
at a predetermined temperature for a predetermined
time to form a solid reduction product; (f) cooling
said solid reduction product; and(g) separating said
solid reduction product into a metallic chromium alloy
phase and a non-metallic phase, wherein steps (a) to
(c) may be performed in any order.
- 3a -
Date Recue/Date Received 2020-07-30
CA0307761320201
W02019/075545
PCT/CA2017/051252
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] The present invention will now be described by
reference to the following figures, in which identical
reference numerals refer to Identical elements and in
which:
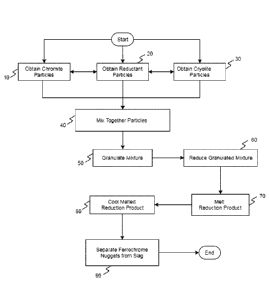
Figure 1 is a flowchart detailing the steps in a
method according to one embodiment of the invention;
Figure 2 shows the temperature profile and the
concentration of evolved gas during heating of a
mixture with a chromite-carbon-cryolite ratio of
100:23:20, heated at 1300 C for two hours;
Figure 3 shows the result after the mixture used for
Figure 2 is reduced and cooled;
Figure 4A shows the concentrate resulting from gravity
separation of the mixture used in Figure 2 after
reduction;
Figure 4B shows the tailings produced by gravity
separation of the mixture used in Figure 2 after
reduction;
Figure 5 shows gas evolution and degree of reduction
curves of a mixture with a chromite-carbon-cryolite
ratio of 100:23:30 in both powdered and pelletized
forms;
Figure 6A shows the powdered mixture used in Figure 5
after reduction and cooling;
Figure 6B shows the pelletized mixture used in Figure
after reduction and cooling;
- 4 -
CA0307761320201
W02019/075545
PCT/CA2017/051252
Figure 7 is a chart showing the effect of pelletizer
press force on metallization rates;
Figure 8 is a chart showing the effect of pelletizer
press force on the size of metallic particles;
Figure 9 shows the temperature profile and the
concentration of evolved gas during heating of three
mixtures with chromite-carbon-additive ratios of
100:23:30;
Figure 10A shows a mixture using bath material (batch
BM1) as the cryolite source after reduction and
cooling;
Figure 10B shows a mixture using bath material (batch
BM2) as the cryolite source after reduction and
cooling;
Figure 11 is a chart showing the effect of different
residence times on the reduction of a powder mixture
at 1300 C;
Figure 12 is a chart showing the effect of different
residence times on the metallic phase weight
percentage of the mixture used in Figure 11;
Figure 13A shows the mixture used in Figure 11 after a
10-minute residence time at 1300 C;
Figure 13B shows the mixture used in Figure 11 after a
60-minute residence time at 1300 C;
Figure 13C shows the mixture used in Figure 11 after a
120-minute residence time at 1300 C;
- 5 -
CA0307761320201
W02019/075545
PCT/CA2017/051252
Figure 13D shows the mixture used in Figure 11 after a
300-minute residence time at 1300 C;
Figure 14 shows the metallic particles concentrated by
magnetic separation of the product forms shown in
Figure 13D;
Figure 15 shows the temperature profile and the
concentration of evolved gas during heating of a
pelletised mixture with a chromite-carbon-cryolite
(BM2) ratio of 100:23:30;
Figure 16 shows the temperature profile, mass loss,
and concentration of evolved gas for powdered mixtures
with chromite-carbon-cryolite ratios of 100:25:20,
100:25:25, and 100:25:30;
Figure 17A shows a powdered mixture with a chromite-
carbon-cryolite ratio of 100:25:20 after reduction;
Figure 17B shows a powdered mixture with a chromite-
carbon-cryolite ratio of 100:25:25 after reduction;
Figure 17C shows a powdered mixture with a chromite-
carbon-cryolite ratio of 100:25:30 after reduction;
Figure 18 shows the mass loss and concentration of
evolved gas for powdered mixtures with a chromite-
carbon-cryolite ratio of 100:25:30 with different
graphite particle sizes;
Figure 19 shows the mass loss and concentration of
evolved gas for powdered mixtures with a chromite-
carbon-cryolite ratio of 100:25:30 with different ore
particle sizes;
- 6 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
Figure 20A shows a powdered mixture with a chromite-
carban-cryolite ratio of 100:25:30 and chromite
particle diameter between 75 pm and 106 pm, after
reduction;
Figure 20B shows a powdered mixture with a chromite-
carbon-cryolite ratio of 100:25:30 and chromite
particle diameter between 75 pm and 90 pm;
Figure 20C shows a powdered mixture with a chromite-
carbon-cryolite ratio of 100:25:30 and chromite
particle diameter between 53 pm and 74 pm;
Figure 21 shows the mass loss and concentration of
evolved gas for powdered mixtures with a chremite-
carbon-oryolite ratio of 100:25:30 with different ore
particle sizes;
Figure 22A shows the mixture used in Figure 21 with
chromite particle diameter between 37 pm and 44 pm
after reduction; and
Figure 228 shows the mixture used in Figure 21 with
chromite particle diameter between 75 pm and 106 pm
after reduction.
DETAILED DESCRIPTION
[0012] In one embodiment of the invention, chromite direct
reduction is accomplished using cryolite as an
additive. Since chromite reduction using cryolite is
a broad process with many potential embodiments, there
are a number of alternatives to practicing the various
embodiments and implementations of the invention,
- V -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
including, for instance, varying the source particle
size.
[0013] One embodiment of the invention is shown in Figure 1.
Figure 1 is a flowchart showing the steps of a method
according to one embodiment of the invention where
chromite is reduced using cryolite as an additive.
First, at step 10, a usable particle form of the
chromite ore is obtained. This is commonly
accomplished by grinding a chromite ore source to the
desired size. At step 20, similarly, particles of
reductant are obtained. Likewise, at step 30,
particles of cryolite are obtained.
[0014] In step 40, all three kinds of particles are mixed
together. Then, in step 50, a granulating unit
creates pellets or briquettes out of the mixture.
Next, in step 60, the pellets or briquettes are
reduced In a furnace, to form a reduction product.
The reduction product is then quickly melted at a
higher temperature than the temperature of reduction
(step 70) to increase the size of ferrochromium
nuggets produced. In step 80, the melted reduction
product is cooled, and then at step 90, the
ferrochromium nuggets are separated from the non-
metallic phase.
[0015] With reference to steps 10, 20, and 30, the kinetics
of reduction mean that certain particle sizes react
more efficiently than others. Thus, these grinding
steps are calibrated to result in specific sizes of
particle. In the case of the chromite ore, the
optimal particle diameter is between 53 pm and 74 pm,
inclusive. However, for practicality, some of the
- 8 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
chromite ore particles may be as large as 150 pm.
(Note that all ranges used herein should be considered
to be inclusive of their end values, unless explicitly
noted otherwise.) Optimal reductant particle diameter
is between 38 pm and 106 pm, though some of the
reductant particles may have diameters of up to 150
pm. While cryolite particles that are less than 106
pm in diameter (preferably less than 63 pm in
diameter) have been found to work with the invention,
it should be noted that individual cryolite particle
size is not as important as the cryolite powder being
fine enough to mix well with the other powdered
material. The cryolite particle size should thus be
such that the cryolite mixes well with the other
powders.
[0016] Additionally, the chromite does not need to be raw
ore. Chromite fines, chromite concentrates or
chromite wastes (for instance, chromite-containing
slags from other ferrcchrome processes or oxides from
flue dusts) may be used instead of raw chromite ore.
The reductant (again, the reactant that takes up
oxygen removed from the chromite) is generally a
widely-available carbon source such as low-ash coke,
graphite or coal.
[0017] Moreover, although it is common to grind the chromite,
reductant, and cryolite individually and in-house, it
should be clear that particles of the desired sizes
can be obtained in any manner (e.g., purchased from
external vendors), without altering the effect of the
invention.
- 9 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
[0018] The cryolite additive may be comprised of pure
cryolite; however, naturally occurring cryolite is
rare and commercially extinct. Pure synthetic
cryolite (synthetic sodium aluminum fluoride) can be
used as a substitute, but impure mixtures containing
cryolite can also be used as the additive.
[0019] In one embodiment of the invention, the cryolite
additive is an impure waste or by-product of aluminium
smelting, known as "bath material". This bath
material is widely available and comprises cryolite
and various other compounds, primarily aluminum
fluoride (A1F3). Cryolite (Na:AlF6) can be considered
a combination of sodium fluoride (NaF) and aluminum
fluoride; thus, a well-known measure called the
"cryolite ratio" represents the relative proportions
of sodium fluoride and aluminum fluoride in bath
material. This measure can be calculated using the
formula in equation (1):
moles of NaF (1)
Cryolite Ratio = ___________________________
moles of A1F3
[0020] Bath material is an impure source of cryolite that is
off from the stoichiometry value (molar ration
NaF/A1F3=3). Nap tends to evaporate from this material
and bath ends up having excess AlF3. Bath material also
contains dissolved alumina and CaF2 as impurities. AlF3
and Al2O3 both have a negative effect on the
effectiveness of bath material as a source of cryolite
for direct reduction. However, bath material
containing up to 11 wt% excess AlF3 and 8% dissolved
A1203 has been found to be acceptable. The cryolite
- 10 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
ratio (equation 1) should be between 1 and 7,
encompassing the typical variation of bath material
produced during aluminum smelting. Specific impurities
in bath material, such as CaF2 (6 wt% of excess of
CaF2), have been found to have a positive effect on
reduction.
[0021] The use of bath material as the source of the cryolite
additive provides several benefits. Not only is bath
material widely available, it is also cost-effective.
Moreover, there is an environmental benefit, as using
bath material in chromite reduction recycles this
hazardous waste product and extends its useful life
before disposal.
[0022] It should be noted that, in step 40, when the
particles are mixed together, the mixture is
proportioned by weight, with the chromite source
particles comprising the largest part of the mixture.
The proportion of chromite to carbon to cryolite can
vary between 100:15:15 and 100:25:30, depending on the
desired application.
[0023] It should also be noted that the optional granulation
step, step 50, may be implemented using a granulating
unit. Such a granulating unit may take the form of,
for example, a compression-molding machine, a disc or
drum pelletizer, or an extruder. The granulating unit
creates pellets or briquettes out of the mixture. The
pellets or briquettes have the same chromite-carbon-
cryolite ratio as the original mixture, and can have
diameters as small as 1 cm and as large as 2 cm. Of
course, this granulating step may be omitted and the
- 11 -
CA 077613 2020--31
W02019/075545
PCT/CA2017/051252
powder mixture can be moved to the reduction step
without further granulating the mixture.
[0024] For the chromite reduction step, step 60, many
different kinds of furnaces may be used, including,
for example, rotary kiln, rotary hearth, tunnel
hearth, multiple hearth, and paired straight hearth.
The reduction reaction, as governed by the furnace,
may include multiple stages, including drying,
preheating, reduction itself, and cooling. Depending
on various factors, the furnace temperature can be as
low as 1200 C or as high as 1400 C.
[0025] Whatever furnace type and furnace temperature are
used, the reduction process requires a reducing
atmosphere. The reducing atmosphere is an atmospheric
condition well known in the art, wherein the removal
of oxidizing gases (including oxygen) prevents
oxidation and encourages chromite reduction (the
removal of oxygen from the chromite). Many materials
and techniques to improve the reducing atmosphere are
known in the field. If the furnace used for reduction
lacks the capacity for built-in atmospheric
adjustment, a carbonaceous atmosphere adjusting agent
may be added to the furnace. Many carbonaceous
materials may be used as the atmosphere adjusting
agent, including, for example, coal, waste plastic,
and biomass. The atmosphere adjusting agent may be
placed under the feedstock (the pellets, briquettes,
or non-granulated mixture) as a bed layer in the
furnace, or it may be added on top of the feedstock to
shelter the feedstock from further oxidation.
- 12 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
[0026] It should be clear that the reducing atmosphere can be
achieved in the furnace by adjusting the air to fuel
ratio of the burner or by purging air from the
chamber. In case the controlled atmosphere is not an
option for the furnace design, a carbonaceous
adjusting atmosphere agent can be added to the mixture
at the reduction stage to control the atmosphere in
the vicinity of the mixture. This adjusting agent can
vary from coal to waste plastic or biomass. This
material can be used as a bed layer for the feedstock
or this material can be used to cover the feedstock to
protect it from further reduction.
[0027] Once the reduction step (step 60) is complete, the
furnace contains the "reduction product": ferrochrome
alloy nuggets and non-metallic phases (reduced
chromite, salt and oxyflcuride phases). If larger
nuggets of ferrochrome are needed, the nugget size can
be increased by quickly melting the reduction product
at high temperatures (step 70). It should be clear
that step 70 is optional and that the reduced product
may be moved directly to the separation stage without
any melting.
[0028] In the event that the melting step is implemented, the
melting unit can be separate from the main reduction
furnace or can be a section of the main reduction
furnace that maintains a temperature between 1350 C
and 1700 C. Although the relatively high temperatures
require more energy, the melting step does not take
long: the residence time of melting can be only ten to
thirty minutes. The short residence time at higher
temperatures means that this process is still more
efficient than conventional smelting processes.
- 13 -
CA 077613 2020--31
W02019/075545
PCT/CA2017/051252
[0029] Before the ferrochrome nuggets can be separated from
the non-metallic phases, the reduction product must be
substantially cooled (step 80) so that it solidifies.
The cooling step cools the melted reduction product
resulting from step 70 to a temperature below 500 C.
This cooling also prevents unwanted oxidation of the
reduction product.
[0030] After cooling, the reduction product is sent to a
separation unit which separates the alloy nuggets from
the non-metallic phases (step 90). The size of the
nuggets may dictate whether a comminution stage is
needed before separation or not. The differences in
the specific gravities and magnetic properties of the
ferrochrome and non-metallic phases mean that well-
known physical separation techniques may be used.
Such techniques include magnetic separation and/or
gravity separation.
[0031] Note that steps 10 to 30 above may be performed in any
order. Additionally, these steps may be performed
simultaneously or at different times. Further, steps
to 30 may be performed in separate locations or the
same location. Steps 10 to 30 may result in large
batches of particles, small batches of particles, or
any combination thereof.
[0032] Also, as noted above, granulation of the mixture is
not a required step in the process. Depending on the
intended application, and the type of reduction
furnace to be used, step 50 may be omitted from the
method. Likewise, as noted above, the melting step 70
is not a necessary step in the invention. Depending
- 14 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
on the intended use of the alloy produced, step 70 may
be omitted.
EXAMPLES
[0033] The following examples show the effects of varying
different parameters of the invention, including the
composition of the feedstock, the residence time
during reduction, whether the mixture is granulated or
not, and the diameter of the chromite and carbon
source particles.
[0034] To ensure that the effects of each parameter could be
seen in isolation, other parameters were kept constant
in testing. A small-scale horizontal tube furnace,
purged with argon gas at a flow rate of 200 ml per
minute, held an alumina crucible containing the
feedstock. The furnace was heated to 1300 C. The
evolution of the furnace atmosphere ("evolved gas")
and the temperature of the feedstock were continuously
measured during each test.
[0035] Tables 1 to 3 below show the chemical composition of
the source components. Two sets of chromite ore
particles were tested, one set having particle
diameters between 75 pm and 106 pm, and the other
having particle diameters between 53 pm and 74 pm.
The composition of the chromite particles is shown in
Table 1.
[0036] Table 2 shows the composition of the carbon reductant
source (graphite, almost entirely carbon but with some
impurities). Table 3 shows the composition of the
three different cryolite sources that were examined:
synthetic cryolite with a cryolite ratio of 3; a batch
- 15 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
of bath material with a cryolite ratio of 2.2; and a
batch of bath material with a cryolite ratio of 2.3.
In each test, the cryolite source was ground into
particles having diameters under 63 pm.
Table 1. Chromite Ore Composition (wt%).
Chromite Cr203 Fe203 Cr/Fe A1203 Si02 Mg0 TiO2 Ni0 Mn0 Ca0
ore
75pm -
43.40 21.22 2.0 12.98 5.45 14.19 0.33 0.18 0.22 0.10
106pm
53pm -
33.20 18.83 2.0 12.26 8.37 16.89 0.30 0.32 0.19 0.14
74pm
Table 2. Carbon Source Composition (wt%).
Carbon C B Al Ca Cu Ni Si V Zn
Graphite 99.99 0.04 0.06 0.01 0.03 0.04 0.04 0.00 0.00
Table 3. Cryolite Source Composition (wt%).
NaF
270
Cryolite NaF A1F3 CaF2 A1203 MgF2 KF P205 Fe203 Excess
AlE3 (mole)
Pure 60.0 40.0 3
Bath
Material 43.9 39.3 5.3 2.6 0.3 0.1 0.01 0.01 10.0 2.2
(BM1)
Bath
Material 46.7 40.8 5.2 1.3 0.2 0.2 0.01 9.7 2.3
(BM2)
- 16 -
CA 077613 2020--31
W02019/075545
PCT/CA2017/051252
Baseline Cryolite Tests
[0037] In the first test performed, the effect of cryolite
was examined in the embodiment of the invention that
does not include either pelletization of the mixture
or melting of the reduction product. Chromite ore
particles with diameters between 75 pm and 106 pm were
mixed together with graphite particles having
diameters between 53 pm and 74 pm. The chromite ore-
carbon-cryolite ratio was 100:23:20. The powdered
mixture was heated to 1300 C in the test furnace and
held at 1300 C for a residence time of two hours.
[0038] Figure 2 is a chart showing the temperature profile of
the mixture and the evolved gas in the furnace in this
first test. As can be seen, the powdered mixture was
steadily heated and settled at 1300 C after
approximately 13500 seconds. The dominant gas
evolution is carbon monoxide (CO), which peaked just
before the 1300 C temperature was reached. The CO
level drops drastically during the two-hour residence
at 1300 C, but some CO remains (approximately 1.7% by
volume), indicating that reduction continues. The
line labelled "Reduction %" passes the 100% mark by
the end of the two-hour reduction time, clearly
showing that the entire sample was reduced.
[0039] The chromium and iron metallization rates of the
reduced sample were then analyzed, and found to be 97%
and 98%, respectively. In the absence of any flux,
these metallization rates are typically between 60 and
70%. This leads to the implication that using the
cryolite flux significantly Increases the
metallization rates.
- 17 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
[0040] Figure 3 is an SEM micrograph image of this first
sample, after reduction. The bright white areas are
the ferrochrome alloy, and the light grey phase is the
residual chromite and the dark grey phase represents
unwanted salt and reduced chromite. As can be seen,
the ferrochrome alloy has formed into relatively large
nuggets at the interfaces of the reductant and the
molten salt phase: analysis of the sample showed a
particle size distribution measure ("grind size" or
P80, a well-known metric in the field) of 68 pm. Later
analysis also showed that the alloy phase contained
between 56% and 63% chromium and only 23% to 24% iron.
[0041] The reduced chromite, moreover, also shows some
internal metallization, with a chemical composition of
44% for chromium and 42% for iron. The degree of
liberation based on the liberation analysis data is
acceptable.
[0042] Gravity separation techniques were applied to this
sample, using a small elutriating tube. Figures 4A
and 4B show the products of a single-stage separation:
Figure 4A shows the concentrated larger nuggets of the
alloy phase and Figure 4B shows the tailing (smaller
pieces of unwanted phases, salt, and gangue). These
results can be improved by multi-stage separation or
other techniques.
Effects of Pelletization
[0043] The effect of pelletization of the mixture was also
examined. Pellets were compared with a powdered
mixture of the same composition and treated under
identical conditions. The mixture used had a chromite-
carbon-cryolite ratio of 100:23:30, with chromite
- 18 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
particles having diameters between 75 pm and 106 pm,
and graphite particles having diameters between 53 pm
and 74 pm. Pellets were created by adding this
mixture to a manual press with a 13 mm die, and
applying four tonnes press force, producing a disc 2
mm in height. Both the powdered mixture and the
pellets were heated to 1300 C for two hours. Figure 5
shows the evolved gas profile of this test, the
furnace temperature, and the percentage reduction.
[0044] As can be seen from Figure 5, the pelletized mixture
outperformed the powdered variant, off-gassing more
carbon dioxide and carbon monoxide and, based on gas
analysis, achieving reduction rates over 100%. Though
the powdered mixture did not reach the same level
(i.e., not quite reaching 90% reduction), the
reduction rates achieved are still significant with
improvements over the 60% to 70% reduction seen in
reduction without cryolite.
[0045] Figures EA and 6B show the results of this test, with
Figure 6A showing the reduced powdered sample and
Figure 6B showing the reduced pelletized sample. It
is evident from the images that pelletization
substantially improves the metallization rate and
increases the size of the metallic alloy nuggets.
Analysis of the samples confirms the advantages of
pelletization: the metallization rates of the
pelletized sample were 97% for chromium and 98% for
iron, while in the powder the rates were only 93% for
chromium and 97% for iron. The Pv size metric for the
alloy nuggets in the powdered sample, further, was
only 70 pm, while the pelletized sample produced alloy
nuggets with a PH metric of 99 pm.
- 19 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
[0046] Thus, it should be clear that compressing the powdered
mixture into pellets produces better results than
leaving it in the powdered form. However, the
powdered form still shows high levels of reduction,
compared to the prior art, and may be preferred for
some applications.
Effects of Pelletizer Press Force
[0047] The effect of varied press force on the reduction of
the pelletized mixture was also examined and the
results are shown in Figures 7 and 8. Figure 7 shows
the relationship between the press force and the
composition by weight of the reduced samples. As can
be seen from Figure 7, as the press force Increases
above 4T, the weight percentage of the metallic alloy
phase decreases substantially and the weight
percentage of the residual chromite phase
correspondingly increases.
[0048] Likewise, Figure 8 shows the relationship between the
press force and the Pc size metric of the alloy
nuggets. It can be seen from Figure 8 that the Ps()
increases relatively smoothly with increasing press
force. At a press force of 10T, the Pso of the sample
was 170 pm. Thus, it is clear that adjusting the
press force alters the end results: depending on the
application, higher or lower press forces may be
preferable.
Use of Cryolite-Containing Bath Material as Additive
[0049] Bath material from aluminum smelting was also examined
as an additive, and compared with pure cryolite. Two
separate batches of bath material were considered,
- 20 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
dubbed "BM1" and "BM2". Their composition is shown in
Table 3. Each mixture was pelletized with chromite
and carbon in a 100:23:30 chromite-carbon-additive
ratio, heated to 1300 C, and held in residence at
1300 C for two hours. Figure 9 shows the gas
evolution and temperature profile for each mixture.
As can be seen, the two bath material sources behaved
relatively similarly. Although the mixture of 30%
pure cryolite produced slightly more carbon monoxide
gas, the bath material sources nevertheless produced
excellent results. Analysis revealed that 96% of the
chromium in batch BM1, and 95% of the chromium in
batch BM2, was successfully metallized, and both
batches had iron metallization rates of 98%. Figure
10A shows batch BM1 after reduction. Figure 10B shows
batch BM2 after reduction. From these images it is
evident that the bath material produces significant
metallization and large metallic alloy nuggets.
Indeed, Ps() size analysis of each sample showed that
batch BM1 alloy nuggets had a P80 metric of 87 pm and
batch BM2 alloy nuggets had a Po metric of 79 pm.
Effects of Residence Time
[0050] Next, the effect of residence time on chrcmite
reduction was examined, using four samples of a
powdered mixture with a chromite-carbon-cryolite ratio
of 100:23:20. (The pure synthetic cryclite was used
here.) Each sample was heated to 1300 C, and reduced
for (respectively) 10 minutes, 1 hour, 3 hours, and 5
hours.
[0051] Figure 11 shows the temperature profiles and reduction
curves of these samples. As can be seen, the sample
- 21 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
reduced for ten minutes only achieved around 60%
reduction. The one-hour reduction produced better
results, plateauing at just under 80%. However, the
three-hour reduction and five-hour reduction were the
most effective, achieving just over 90% reduction for
the three-hour reduction and just under 100% reduction
for the five-hour reduction.
[0052] Residence time also affected the weight percentage of
the reduced samples and the size distribution of the
alloy nuggets. As shown in Figure 12, both the
percentage weight of the alloy phase and the particle
size substantially increased between the one-hour
residence time and the two-hour residence time,
suggesting that longer residence times would be
preferable. However, the difference between the two-
hour reduction and the five-hour reduction was not as
pronounced. Thus, when accounting for the greater
energy costs of longer residence times, a two-hour
reduction may well be preferred over longer times.
[0053] The reduced samples from these tests are shown in
Figures 13A-13D. Figure 13A, an image of the sample
reduced for only 10 minutes, shows some reduction
(bright white area), but much less than other tests.
The metallization percentage increases with time, as
can be seen: there is more of the bright white area in
Figure 13B than in Figure 13A, and even more in Figure
13C than in Figure 13B. However, Figure 13D, though
having slightly more metallization than Figure 13C,
does not show dramatically greater metallization.
[0054] Table 4 shows the chemical composition by weight of
the metallic alloy nuggets formed in each of these
- 22 -
CA0307761320201
W02019/075545 PCT/CA2017/051252
four samples shown in Figures 13A-13D. The weight
percentage of chromium and iron is given, as is the
weight percentage of silicon. As can be seen, each
of the samples has a high ratio of chromium to iron
(between approximately 2.2 and approximately 2.4).
Table 4. Alloy Nugget Composition by Weight for Varied
Residence Times.
Chromium Weight Iron Weight Silicon
Weight
Residence Time
Percentage Percentage Percentage
minutes 56 22 0
60 minutes 60 27 < 1
120 minutes 57 24 < 1
300 minutes 60 25 0.5
[0055] The result of magnetic separation of the metallic
phase formed after 5 hr. reduction from the gangue
materials after magnetic separation is shown in Figure
14. This image illustrates a perfect separation after
two cycles of magnetic separation. Residence time
evaluations were also performed for mixtures using a
bath material source as the additive (specifically,
batch BM2). Figure 15 shows the evolved gas profiles,
the furnace temperature, and the reduction curves for
a pelletized mixture with a chromite-carbon-cryolite
ratio of 100:23:30, where the cryolite is batch BM2
bath material, reduced for 2 hours and for 5 hours.
[0056] Again, the difference between two-hour reduction and
five-hour reduction, though evident, is not so
pronounced as to render a two-hour residence time
useless. After two hours, 89% of the sample was
- 23 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
reduced. After five hours, the reduction had reached
100%. Additionally, analysis showed that the weight
percentage of the residual chromite phase was 6.7%
after two hours, and more than halved (2.4%) after
five hours. Further, increasing the residence time
from two hours to five hours increased the P,,) metric
from 79 pm to 111 pm. However, again, the greater
energy input needed for five-hour reduction may offset
its advantages over a two-hour reduction.
Effects of Cryolite Concentration in Mixture
[0057] The effect of varying the cryolite concentration was
examined by testing three different powdered mixtures.
The first mixture had a chromite-carbon-cryolite ratio
of 100:25:20. The second mixture had a chromite-
carbon-cryolite ratio of 100:25:25. The third mixture
had a chromite-carbon-cryolite ratio of 100:25:30.
Figure 16 shows the temperature profile and evolved
carbon monoxide from each sample, as well as the mass
loss from thermogravimetric analysis. The mass loss,
though correlated with the degree of reduction, is
subject to other factors, and thus an analysis of the
evolved gas is generally a better predictor of
reduction success. As can be seen, though the mixture
with only 20% cryolite is out-performed by both the
25% cryolite and 30% cryolite mixtures, there is no
significant difference between the reduction levels in
the 25% mixture and the 30% mixture.
[0058] From Figures 17A-17C, it can be seen that complete
reduction was achieved with each of the three
mixtures. Figure 17A, illustrating the reduced 20%
cryolite mixture, shows relatively large metallic
- 24 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
alloy nuggets and no unreacted phase. The same can be
said for Figures 17B and 17C (with Figure 17B showing
25% cryolite and Figure 17C showing 30% cryolite).
Differences between each figure are not readily
apparent to the naked eye. Thus, while mixtures with
cryolite concentrations between 25% and 30% by weight
may be optimal, mixtures with cryolite concentrations
as low as 20% may also be useful for some
applications.
Effect of Graphite Particle Size
[0059] Graphite particle size was varied to examine its
effects on chromite reduction. Five different
mixtures were tested, each using chromite particles of
diameters between 75 pm and 106 pm and having a
chromite-carbon-cryolite ratio of 100:25:30. Pure
synthetic cryolite was also used (as opposed to bath
material).
[0060] Figure 18 shows the mass loss, temperature profile,
and evolved carbon monoxide for each set of graphite
particles from thermogravimetric measurement. Table 5
shows the same data in numerical form. As can be
seen, the mass loss curves are very similar. There is
slightly more variance in the carbon monoxide lines:
the graphite particles with diameters between 106 pm
and 150 pm out-perform most of the smaller sets, but
the set of particles with diameters between 53 pm and
106 pm was most effective and produced the highest
carbon monoxide peak. Thus, experimental results
suggest that the optimal graphite particle diameter is
between 53 pm and 106 pm.
- 25 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
Table 5. Mass Loss and CO Gas Evolution with Varied Graphite
Particle Size.
(Mass Loss) -
Graphite Total
Carbon
% Mass Loss / (Mass Loss from
Particle Monoxide
(CO)
1 mg Chromite H20 Evaporation) -
Size Gas Intensity
(Mass of Cryolite)
53 pm -
68.77 35.87 4.220E-05
75 pm
38 pm -
68.79 36.29 3.990E-05
45 pm
75 pm -
68.07 35.37 3.950E-05
106 pm
106 pm -
68.3836 35.38 6.630E-05
150 pm
53 pm -
70.1852 37.19 9.050E-05
106 pm
Effect of Chromite Ore Particle Size
[0061] In these tests, the size of the chromite ore particles
was varied, to examine the effect of chromite ore
particle size on chromite reduction. Additionally,
two sets of graphite particles were used (one set
having diameters between 53 pm and 75 pm, the other
having diameters between 105 pm and 150 pm) to examine
any potential interaction between particles of
different sizes. The composition of each tested
mixture is shown in Table 6.
- 26 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
Table 6. Mixture Composition with Varied Particle Size.
Graphite Chromite
Chromite-Carbon-
Mixture Particle Size Particle Size
Cryolite Ratio
(1-1m) (1-1m)
A.1 53 - 75 53 - 74 100:25:30
A.2 53 - 75 75 - 90 100:25:30
A.3 53 - 75 75 - 106 100:25:30
B.4 105 - 150 37 - 44 100:25:30
B.5 105 - 150 75 - 105 100:25:30
[0062] Figure 19 shows the temperature profile, the mass loss
curves, and the carbon monoxide profiles for mixtures
A.1, A.2, and A.3 (as defined in Table 6) from
thermogravimetric measurements. As the concentrations
of chromite and iron oxide (which are relevant to mass
loss) necessarily change with chromite particle size,
the mass loss data shown in Figure 19 was normalized
per mole of (Cr+Fe)-that is, per mole of combined
chromium and iron. Table 7, additionally, shows
normalized mass loss and carbon monoxide data.
Table 7. Mass Loss and CO Gas Evolution with Varied Chromite
Particle Size.
(Mass Loss) -
Total Carbon
% Mass Loss / (Mass Loss from
Mixture Monoxide (CO)
mole (Cr+Fe) H20 Evaporation) -
Gas Intensity
(Mass of Cryolite)
A.1 97.8 62.3 5.840E-05
A.2 89.51 56.2 3.110E-05
A.3 82.81 49.2 3.010E-05
- 27 -
CA 077613 2020--31
W02019/075545
PCT/CA2017/051252
[0063] As can be seen from the CO curves and the intensity
data, mixture A.1 (with the smallest chromite particle
sizes) was more effective for reduction than mixtures
with larger chromite particles. This is also evident
from Figures 20A-20C, which show mixtures A.3, A.2,
and A.1, respectively. It is clear from these images
that greater levels of reduction are achieved with
mixture A.1 (Figure 20C): there is substantially less
of the unreduced core phase in each image as the
particle size is decreased.
[0064] Finally, varied chromite particle size was examined in
mixtures B.4 and B.5 (which use larger graphite
particles than the "A" mixtures of Table 6). Again,
as can be seen from Figure 21, which shows the
temperature profile, evolved gas, and mass loss during
reduction of mixtures B.4 and B.5, the mixture with
smaller chromite particles (B.4) achieved higher
reduction levels than the mixture with larger chromite
particles. Figures 22A and 22B, showing the reduced
mixtures B.4 and B.5, respectively, confirm this
analysis. Thus, it is apparent that smaller chromite
particles improve the effectiveness of the reduction
process no matter the size of the reductant particles.
[0065] A better understanding of the present invention may be
obtained by consulting the following references:
[1] F. Winter, "Production of Chromium Iron Alloys
Directly from Chromite Ore," US Patent Publication
US2016/0244864 Al, 2016.
[2] H. G. Katayama, M. Tokuda, and M. Ohtani, "Promotion
of the Carbothermic Reduction of Chromium Ore by the
Addition of Borates," Tetsu-to-Hagane (Journal Iron Steel
- 28 -
CA 03077613 2020-03-31
W02019/075545
PCT/CA2017/051252
Inst. Japan), vol. 72, no. 10, pp. 1513-1520, 1986.
[3] K. Bisaka, M. 0. Makwarela, and M. W. Erweel,
"Solid-State Reduction of South African Manganese and
Chromite Ores," in IMPC 2016, 2016, pp. 1-16.
[4] W. K. Lu, "Process of the production and refining of
low-carbon DRI(direct reduced iron)," PCT Patent
publication W02012149635A1, 2012.
[5] A. Lekatou and D. Walker, "Effect of silica on the
carbothermic reduction of chromite," Ironmak. Steelmak.,
no. May, p. 133, 1997.
[0066] A person understanding this invention may now conceive
of alternative structures and embodiments or
variations of the above all of which are intended to
fall within the scope of the invention as defined in
the claims that follow.
- 29 -