Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR DESULFURIZING PETROLEUM FEEDSTOCKS
TECHNICAL FIELD
[0003] The present
invention relates to a process for removing nitrogen, sulfur,
and heavy metals from sulfur-, nitrogen-, and metal-bearing shale oil,
bitumen, or
heavy oil. More particularly, the invention relates to a method of
regenerating alkali
metals and sulfur from sulfides and polysulfides that were obtained from the
sulfur-,
nitrogen-, and metal-bearing shale oil, bitumen, or heavy oil.
1
CA 2863357 2018-01-10
BACKGROUND
[0004] U.S. Patent Application Serial No. 12/916,984 has been published
as
United States Patent Application Publication No. 2011/0100874. The reader is
presumed to be familiar with the disclosure of this published application.
This
published application will be referred to herein as the "1874 application."
[0005] U.S. Patent No. 8,088,270 relates to a "Process For Recovering
Alkali
Metals And Sulfur From Alkali Metal Sulfides And PolySulfides." The reader is
presumed to be familiar with the disclosure of this published patent. This
published
patent will be referred to herein as the "270 patent."
[0006] The demand for energy and the hydrocarbons from which that energy
is derived is continually rising. The hydrocarbon raw materials used to
provide this
energy, however, can contain difficult to remove sulfur and metals that hinder
their
usage. Sulfur can cause air pollution, and can poison catalysts designed to
remove
hydrocarbons and nitrogen oxide from motor vehicle exhaust. Similarly, other
(heavy) metals contained in the hydrocarbon stream can poison catalysts
typically
utilized for removal of sulfur.
[0007] Extensive reserves of shale oil exist in the U.S. that will
increasingly
play a role in meeting U.S. energy needs. Over 1 trillion barrels reserves lay
in a
relatively small area known as the Green River Formation located in Colorado,
Utah,
and Wyoming. As the price of crude oil rises, these shale oil resources become
more attractive. However, technical issues surrounding this shale oil remain
to be
solved. For example, this shale oil has a relatively high amount of nitrogen
contained therein (in addition to high levels of heavy metals and sulfur).
Shale oil
2
CA 2863357 2018-05-30
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
characteristically is high in nitrogen, sulfur, and heavy metals which makes
subsequent hydrotreating difficult. According to America's Strategic
Unconventional
Fuels, Vol. 111--Resource and Technology Profiles, p. 111-25, nitrogen is
typically
around 2% and sulfur around 1% in most samples of shale oil (Heavy metals are
also present.) Heavy metals contained in shale oil pose a large problem to
upgraders trying to upgrade this shale oil for commercial use. For example,
sulfur
and nitrogen typically are removed from the shale oil via hydrotreating at
elevated
temperatures and pressures using catalysts such as Co--Mo/A1203 or Ni--
Mo/A1203.
However, such catalysts are deactivated (poisoned) by the presence of heavy
metals
as the heavy metals operate to mask the catalysts.
[0008] Another example of a source of hydrocarbon fuel where the
removal of sulfur poses a problem is in bitumen existing in ample quantities
in
Alberta, Canada and heavy oils such as in Venezuela. In order to remove
sufficient
sulfur from the bitumen for it to be useful as an energy resource, excessive
hydrogen
must be introduced under extreme conditions, which creates an inefficient and
economically undesirable process.
[0009] Over the last several years, sodium has been recognized as being
effective for the treatment of high-sulfur petroleum oil distillate, crude,
heavy oil,
bitumen, and shale oil. Sodium is capable of reacting with the oil and its
contaminants to dramatically reduce the sulfur, nitrogen, and metal content
through
the formation of sodium sulfide compounds (sulfide, polysulfide and
hydrosulfide).
Examples of the processes can be seen in U.S. Patent Nos. 3,785,965;
3,787,315;
3,788,978; 4,076,613; 5,695,632; 5,935,421; and 6,210,564. This process is
further
described in the '874 application.
3
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0010] When shale oil, heavy oil or bitumen or other oil feedstock is
reacted with the alkali metals, this reaction occurs generally at a
temperature
between 150 - 450 C. This reaction is also performed at a pressure that is
anywhere between atmospheric pressure and 2000 psi. For example 2 moles alkali
metal and 1 mole hydrogen (H2) may be needed per mole sulfur according to the
following initial reaction:
R¨S¨R' + 2M + H2 -> R¨H + R'¨H + M2S,
Where M is an alkali metal such as sodium or lithium and 3 moles alkali metal
and 1.5 moles hydrogen (H2) may be needed per mole nitrogen according to the
following initial reaction:
R,R',R"¨N + 3M + 1.5H2 ¨> R¨H + R'¨H + R"¨H + M3N
Alternatively, the '874 application describes a method of upgrading an oil
feedstock
(such as heavy oil, shale oil, bitumen, etc.) by combining the oil feedstock
with an
alkali metal and an upgradant hydrocarbon material. This reaction operates to
remove the sulfur, nitrogen and/or heavy metals contained within the oil
feedstock.
[0011] It should also be noted that heavy metals contained in the shale
oil
may also be removed via the use of alkali metals such as sodium. Heavy metals
contained in organometallic molecules such as complex porphyrins are reduced
to
the metallic state by the alkali metal. Once the heavy metals have been
reduced,
they can be separated from the oil because they no longer are chemically
bonded to
the organic structure. In addition, once the metals are removed from the
porphyrin
structure, the nitrogen heteroatoms in the structure are exposed for further
denitrogenation.
[0012] The following is a summary of the reaction of shale oil, bitumen
and/or other oil hydrocarbons when they are reacted with alkali metals, such
as
4
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
lithium or sodium. Liquid phase alkali metal is brought into contact with the
organic
molecules containing heteroatoms and metals in the presence of hydrogen,
methane, and also gases such as nitrogen (or inert gases such as helium, neon,
argon, krypton, xenon and radon). The free energy of reaction with organic
sulfur,
organic nitrogen and organic heavy metals is stronger with alkali metals than
with
hydrogen, so the reaction more readily occurs without full saturation of the
organics
with hydrogen. (Hydrogen is generally used in the reaction to cap broken bonds
previously attached to heteroatoms and metals, prevent carbon-carbon bonds
from
forming or coking.) Once the alkali metal compounds are formed and heavy
metals
are reduced to their metallic states, it is necessary to separate these
products from
the hydrocarbon materials. A gravimetric separation, such as centrifugation or
filtering, can separate the organic, upgraded oil, from the salt phase,
metallic phase,
and organic solids which may be formed.
[0013] Once the alkali metal sulfide has been separated from the oil,
sulfur
and metals are substantially removed, and nitrogen is moderately removed.
Also,
both viscosity and density are reduced, while the API gravity is increased.
Bitumen
or heavy oil would be considered synthetic crude oil (SCO) and can be shipped
via
pipeline for further refining. Similarly, shale oil will have been
considerably upgraded
after such processing. Subsequent refining will be easier since the
troublesome
metals have been removed.
[0014] Although the effectiveness of the use of alkali metals such as
sodium in the removal of sulfur has been demonstrated, the process is not
commercially practiced because a practical, cost-effective method to
regenerate the
alkali metal has not yet heretofore been proposed. Several researchers have
proposed the regeneration of sodium using an electrolytic cell, which uses a
sodium-
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
ion-conductive beta-alumina membrane. Beta-alumina, however, is both expensive
and fragile, and no significant metal production utilizes beta-alumina as a
membrane
separator. Further, the cell utilizes a sulfur anode, which results in high
polarization
of the cell causing excessive specific energy requirements.
[0015] Metallic sodium is commercially produced almost exclusively in a
Downs-cell such as the cell described in U.S. Patent No. 1,501,756. Such cells
electrolyze sodium chloride that is dissolved in a molten salt electrolyte to
form
molten sodium at the cathode and chlorine gas at the anode. The cells operate
at a
temperature near 600 C, a temperature compatible with the electrolyte used.
Unlike
the sulfur anode, the chlorine anode is utilized commercially both with molten
salts
as in the co-production of sodium and with saline solution as in the co-
production of
sodium hydroxide.
[0016] Another cell technology that is capable of producing sodium metal
at a temperature of less than 200 C has been disclosed by Jacobsen et al. in
U.S.
Patent No. 6,787,019, and Thompson et al. in U.S. Patent No. 6,368,486. In
those
disclosures, low temperature co-electrolyte is utilized with the alkali halide
to form a
low temperature melting electrolyte.
[0017] Accordingly, the present embodiments are designed to provide a
cost-effective and efficient method for the regeneration of alkali metals used
in the
desulfurization, denitrogenation, and demetallation of hydrocarbon streams. As
will
be described herein, the present invention is able to remove contaminants and
separate out unwanted material products from
desulfurization/denitrogenation/demetallation reactions, and then recover
those
materials for later use.
6
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
SUMMARY
[0018] The present embodiments relate to a denitrogenation and
desulfurization technology that is insensitive to the heavy metal content and
at the
same time demetallizes very effectively. The deep demetallization provides an
enormous benefit because additional hydrotreating processes will not be
affected by
the metals originally contained in the shale oil and tar sands.
[0019] The present embodiments provide a process for removing nitrogen,
sulfur, and heavy metals from sulfur-, nitrogen-, and metal-bearing petroleum
feedstocks such as shale oil, bitumen, coker diesel or heavy oil. The present
embodiments further provide an electrolytic process of regenerating alkali
metals
from sulfides, polysulfides, nitrides, and polynitrides of those metals. The
present
embodiments further provide an electrolytic process of removing sulfur from a
polysulfide solution.
[0020] One non-limiting embodiment within the scope of the invention
includes a process for oxidizing alkali metal polysulfides electrochemically.
The
process utilizes an electrolytic cell having an alkali ion conductive membrane
configured to selectively transport alkali ions, the membrane separating an
anolyte
compartment configured with an anode and a catholyte compartment configured
with
a cathode. An anolyte is introduced into the anolyte compartment. The anolyte
includes an alkali metal sulfide species and an anolyte solvent that dissolves
alkali
metal sulfide species. A catholyte is introduced into the catholyte
compartment. The
catholyte may be comprised of molten alkali metal or may include alkali metal
ions
and a catholyte solvent. The catholyte solvent may include one of many non-
aqueous solvents such as tetraglyme, diglyme, dimethyl carbonate, dimethoxy
ether,
propylene carbonate, ethylene carbonate, diethyl carbonate. The catholyte may
also
7
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
include an alkali metal salt such as an iodide or chloride of the alkali
metal. Applying
an electric current to the electrolytic cell oxidizes sulfur in the anolyte
compartment to
form elemental sulfur, causes alkali metal ions to pass through the alkali ion
conductive membrane from the anolyte compartment to the catholyte compartment,
and reduces the alkali metal ions in the catholyte compartment to form
elemental
alkali metal.
[0021] Sulfur has higher specific gravity than the anolyte and is easily
separated from the anolyte by gravimetric means, centrifugal separation or may
be
recovered by removing a portion of the anolyte solution from the anolyte
compartment, cooling the removed anolyte solution to precipitate solid phase
sulfur
from the anolyte solution, separating the precipitated sulfur from the anolyte
solution.
In the preferred embodiment, the cell is operated at 115 C or greater such
that the
sulfur formed at the anode is in the liquid phase. If the alkali metal is
sodium, then
the sodium formed at the cathode is also liquid phase.
[0022] By operating the cell at a temperature below the melting
temperature of the alkali metal (e.g., if, for example, if the alkali metal is
lithium),
elemental alkali metal will plate onto the cathode. The cathode may be
periodically
withdrawn from the catholyte compartment to remove the alkali metal.
Alternatively,
in one embodiment within the scope of the invention, the cathode may be
configured
as a flexible band which continuously or semi-continuously loops from inside
the
catholyte compartment to outside the catholyte compartment and electrolytic
cell
housing, enabling the alkali metal to be continuously scraped or removed from
the
cathode.
[0023] The present invention may provide certain advantages, including
but not limited to the following:
8
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
= Operating an electrolytic cell to process an alkali metal sulfide or
polysulfide at temperatures below the melting temperature of the alkali
metal;
= Operating an electrolytic cell continuously or semi-continuously to
process an alkali metal sulfide or polysulfide at temperatures below the
melting temperature of the alkali metal;
= Removing an alkali metal continuously or semi-continuously in solid
form from the cell;
= Removing high alkali metal polysulfides and dissolved sulfur
continuously or semi-continuously from the electrolytic cell;
Separating sulfur continuously or semi-continuously from a stream containing a
mixture of solvent, sulfur, and alkali metal polysulfides such that the
solvent and
alkali metal polysulfides are substantially recovered such that they can be
returned
back to an electrolytic process; and
BRIEF DESCRIPTION OF THE DRAWINGS
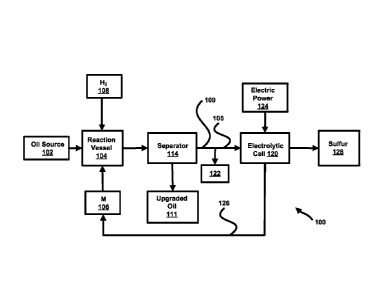
[0024] Figure 1 shows an overall process for upgrading an oil feedstock
that removes nitrogen, sulfur, and heavy metals from sulfur-, nitrogen-, and
metal-
bearing oil sources using an alkali metal and regenerates the alkali metal;
[0025] Figure 2 shows a schematic cross-section of an electrolytic cell
that
may be used to regenerate the alkali metal and sulfur used to react with
sulfur-,
nitrogen-, and metal-bearing oil sources;
[0026] Figure 3 shows a schematic of a process for upgrading the oil and
regenerating the sulfur and alkali metal;
[0027] Figure 4 shows a schematic of an apparatus which can process
electrolytic cell anolyte to extract sulfur;
9
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0028] Figure 5
shows a schematic of another embodiment of a process
for upgrading the oil and regenerating the sulfur and alkali metal; and
[0029] Figure 6
shows a schematic of another embodiment of a process
for upgrading the oil and regenerating the sulfur and alkali metal; and
[0030] Figure 7
shows a schematic drawing of a process for upgrading the
oil feedstock that includes post-treating the petroleum liquid.
DETAILED DESCRIPTION
[0031] The
present embodiments of the present invention will be best
understood by reference to the drawings, wherein like parts are designated by
like
numerals throughout. It will be readily understood that the components of the
present
invention, as generally described and illustrated in the figures herein, could
be
arranged and designed in a wide variety of different configurations. Thus, the
following more detailed description of the embodiments of the methods and
cells of
the present invention, as represented in the Figures, is not intended to limit
the
scope of the invention, as claimed, but is merely representative of present
embodiments of the invention.
[0032] The
overall process is shown schematically in Figure 1 of one non-
limiting embodiment for removing nitrogen, sulfur, and heavy metals from
sulfur-,
nitrogen-, and metal-bearing oil sources using an alkali metal and for
regenerating
the alkali metal. In the process 100 of Figure 1, an oil source 102, such as
high-
sulfur petroleum oil distillate, crude, heavy oil, bitumen, or shale oil, is
introduced into
a reaction vessel 104. As described above, this oil source 102 may have heavy
metals, sulfur and/or nitrogen containing compounds within the oil feedstock
102. An
alkali metal (M) 106, such as sodium or lithium, is also introduced into the
reaction
vessel 104, together with a quantity of hydrogen gas 108 or other gas which
may cap
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
the radicals formed when the bonds with heteroatoms, and metals are broken.
The
alkali metal 106 and hydrogen 108 react with the oil source 102 and its
contaminants
to dramatically reduce the sulfur, nitrogen, and metal content through the
formation
of sodium sulfide compounds (sulfide, polysulfide and hydrosulfide) and sodium
nitride compounds.
[0033] The alkali metal (M) and hydrogen react with the oil 102 at about
300 -400 C and 300-2000 psi according to the following initial reactions:
[0034] R¨S¨R' + 2M + H2 ¨> R¨H + R'¨H + M2S, and
[0035] R,R',R"¨N + 3MNa + 1.5H2 ¨> R¨H + R'¨H + R"¨H + MNa3N
[0036] Where M is an alkali metal such as sodium or lithium and
where R, R', R" represent portions of organic molecules or organic rings.
[0037] Solids from the reaction of alkali metal with petroleum feedstocks may
be
separated in numerous ways including gravimetric, centrifugal methods, and
filtering.
Such separation of the solids may be conducted within a separator 114. The
upgraded oil product 111, which has reduced amounts of heavy metals, sulfur
and
nitrogen containing compounds, may be obtained from the separator 114.
[0038] The solids may be washed with a light petroleum substance such as
hexane, heptane, toluene or mixtures of these substances, or natural gas
condensate, other hydrocarbon liquids, or the like to remove adhered liquid
product.
The light petroleum substance may be stripped away by distillation for example
to
leave behind product liquid that is re-added to the upgraded oil. The light
petroleum
substance may be reused for further washing of solids.
[0039] Solids separated from the petroleum reacted with alkali metal typically
are
a mixture of organic and inorganic constituents. To facilitate separation of
the
organic from inorganic and to prevent adverse reactions and resistive coating
of
11
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
electrodes and membranes, the solids may be treated by heating in the
substantial
absence of oxygen or water. Such heating may occur, for example under the
presence of nitrogen, or hydrocarbon gases such as methane. Such heating may
involve heating to a temperature above 400 C and preferably above 500 C.
During
this heating process, light gases are formed and may be recovered. (These
gases
may be, for example, methane or other hydrocarbons.) This heating process may
be
referred to as "heat treating" 109.
[0040] Following this heat treating process 109 (and subsequent cooling), the
alkali metal sulfides found in the solid materials may be dissolved in solvent
such as
formamide, methyl formamide, dimethyl formamide, acetamide, methyl acetamide,
dimethyl acetamide, ethylene glycol, propylene glycol, 1,2-ethanediol, 1,2-
propanediol, propylene carbonate, ethylene carbonate, diethyl carbonate, N-
methyl
pyrrolidone, tetraethylene glycol dimethyl ether (tetralglyme), acetonitrile,
dimethyl
sulfoxide, liquid ammonia, methyl amine or 1,3-Dimethy1-3,4,5,6-tetrahydro-
2(1H)-
pyrimidinone (DMPU) or combinations of the above. Once
dissolved, any
undissolved portion of solids 122 may be removed by filtration or centrifugal
means.
These undissolved solids may be rich with metals that were originally in the
petroleum feedstock. The
dissolved sulfides may be fed into the anolyte
compartment of the electrolytic cell.
[0041] The solid material dissolved in the solvent (which includes the alkali
metal
sulfides, hydrogen sulfides or polysulfides), as shown by arrow 105, may be
further
processed in an electrolytic cell 120 to remove and recover sulfur and to
remove and
recover the alkali metal. (One example of this type of electrolytic cell 120
is shown in
Figure 2.)
12
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0042] The electrolytic cell 120 receives a solution of the alkali sulfide or
polysulfide in a solvent such as formamide, methyl formamide, dimethyl
formamide,
acetamide, methyl acetamide, dimethyl acetamide, ethylene glycol, propylene
glycol,
1,2-ethanediol, 1,2-propanediol, propylene carbonate, ethylene carbonate,
diethyl
carbonate, N-methyl pyrrolidone, tetraethylene glycol dimethyl ether
(tetralglyme),
acetonitrile, dimethyl sulfoxide, liquid ammonia, methyl amine, or 1,3-
Dimethy1-
3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU) or combinations of the above.
Under
the influence of a source electric power 124, alkali metal ions are reduced to
form
the alkali metal (M) 126, which may be recovered and used as a source of
alkali
metal 106. Sulfur 128 is also recovered from the process of the electrolytic
cell 120.
A detailed discussion of one possible electrolytic cell that may be used in
the
process within the scope of the present invention is described in conjunction
with
Figures 2, 5 and 6.
[0043] It should be noted that the treatment of the solid material by heating
before
dissolving in the polar solvent may be beneficial to the overall process for
upgrading
the petroleum product. If this "heat-treating" 109 of the solid is not
performed, when
the materials are added to the electrolytic cell 120, the electrolytic cell
will ultimately
be "gummed up" or failed. Specifically, organic materials that are present in
the
materials, if not removed via heat treating, will be deposited on the
electrodes,
thereby causing the electrodes to fail. However, by heating the solids in the
manner
described above, the organic materials that would normally fail the electrodes
are
removed (such as through conversion into methane or another gaseous product).
Thus, by heat-treating 109 the solids in the manner outlined herein,
significant
advantages may be obtained.
13
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0044] Figure 2 shows a schematic cross-section of an electrolytic cell 200
which
utilizes many of the features within the scope of the invention. As described
above,
after "heat treating" 109 the solid material (and removing the solids), the
liquid
containing dissolved sodium and sulfides may be added to an electrolytic cell.
Figure 2 shows one example of this type of cell that will receive the "heat
treated"
liquid.
[0045] As shown in Figure 2, electrolytic cell housing 202 is constructed to
enclose
a liquid solvent mixture. The material of construction preferably is an
electrically
insulative material such as most polymers. The material also is preferably
chemically resistant to solvents. Polytetrafluoroethylene (PTFE) is
particularly
suitable, as well as Kynar (which is a commercially available synthetic
resin),
polyvinylidene fluoride, or high density polyethylene (HDPE). The cell housing
202
may also be fabricated from a non-insulative material and non-chemically
resistant
materials, provided the interior of the housing 202 is lined with such an
insulative and
chemically resistant material. Other suitable materials would be inorganic
materials
such as alumina, silica, alumino-silicate and other insulative refractory or
ceramic
materials.
[0046] The internal space of housing 202 is divided into a catholyte
compartment
204 and anolyte compartment 206 by a divider 208. The divider 208 preferably
is
substantially permeable only to cations and substantially impermeable to
anions,
polyanions, and dissolved sulfur. The divider 208 may be fabricated in part
from an
alkali metal ion conductive material. If the metal to be recovered by the cell
is
sodium, a particularly well suited material for the divider is known as
NaSICON
which has relatively high ionic conductivity at room temperature. A typical
NaSICON
composition substantially would be Na1+xZr2SixP3_x012 where 0<x<3. Other
14
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
NaSICON compositions are known in the art. Alternatively, if the metal to be
recovered in the cell is lithium, then a particularly well suited material for
the divider
would be lithium titanium phosphate (LTP) with a composition that is
substantially,
Li(l,õ4y)A1xTi(l_x_y)(PO4)3 where 0<x<0.4, 0<y<0.2. Other suitable materials
may be
from the ionically conductive glass and glass ceramic families and have the
general
composition Li1,AlxGe2PO4. Other lithium conductive materials are known in the
art. The divider 208 may have a portion of its thickness which has negligible
through
porosity such that liquids in the anolyte compartment 206 and catholyte
compartment
204 cannot pass from one compartment to the other, but substantially only
alkali ions
(M+) 210, such as sodium ions or lithium ions, can pass from the anolyte
compartment 206 to the catholyte compartment 204. The divider may also be
comprised in part by an alkali metal conductive glass-ceramic such as the
materials
produced by Ohara Glass of Japan.
[0047] The anode 212 is located within the anolyte compartment 206. It may be
fabricated from an electrically conductive material such as stainless steel,
nickel,
iron, iron alloys, nickel alloys, and other anode materials known in the art.
The
anode 212 is connected 214 to the positive terminal of a direct current power
supply.
The anode 212 may be a mesh, monolithic structure or may be a monolith with
features to allow passage of anolyte through the anode structure. Anolyte
solution is
fed into the anolyte compartment through an inlet 216 and passes out of the
compartment through and outlet 218. The electrolytic cell 200 can also be
operated
in a semi-continuous fashion where the anolyte compartment is fed and
partially
drained through the same passage.
[0048] The electronically conductive cathode 220 is in the form of a strip or
band
that has a portion within the catholyte compartment 204 and a portion outside
the
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
catholyte compartment 204 and cell housing 202, such that the alkali metal 222
can
plate onto the cathode 220 while it is in the catholyte compartment 204. The
alkali
metal 222 can be stripped off the cathode while it is outside the catholyte
compartment. Rotating rollers 224 can define the path of the cathode 220 where
the
path passes near the divider 208 in the catholyte compartment 204, exits the
housing 202, passes through a section where the alkali metal is removed from
the
cathode band 220, then re-enters the housing and returns near the divider 208.
One
or more of the rollers may be driven by a motor or driving mechanism (not
shown) to
cause the cathode 220 to move through an opening 226 in the housing 202 and
pass
out of the housing continuously, semi-continuously or periodically.
[0049] One or more of the rollers may be attached to tensioning devices 228 to
allow the cathode 220 to remain at an acceptable level of tension as the
cathode
band expands or contracts with temperature fluctuations and strains from
stress.
Wiping seals 230 remove catholyte solution from the cathode 220 as it egresses
the
cell so that the catholyte is returned back to the catholyte compartment. The
cathode band may be fabricated from steel, flexible metal alloys, and other
conductive materials suitable for its intended purpose. A scraper 232 can be
used to
remove the plated alkali metal 222 from the cathode 220 as it moves.
Alternatively,
the cathode may be exposed to a heated zone 234 that melts the alkali metal
off of
the cathode 220. The removed alkali metal 236 may fall into a container 238
which
may have a conveyance system (not shown) to transfer the alkali metal 236 away
from the cell 200 to a storage area or point of use.
[0050] The cathode 220 is polarized by a connection 240 to the negative
terminal
of a power supply. This connection may be made with an electronically
conductive
brush 242 that contacts the cathode 220 or it may be made through one or more
of
16
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
the rollers 224 contacting the cathode belt. The catholyte compartment 204 may
have an inlet port 244 and an outlet port 246 to transfer catholyte solution
in and out
of the catholyte compartment 204 when required.
[0051] Within the catholyte compartment is an alkali ion conductive liquid
which
may include a polar solvent. Non-limiting examples of suitable polar solvents
are
tetraglyme, diglyme, dimethyl carbonate, dimethoxy ether, propylene carbonate,
ethylene carbonate, diethyl carbonate and such. An appropriate alkali metal
salt,
such as a chloride, bromide, iodide, perchlorate, hexafluorophosphate or such,
is
dissolved in the polar solvent to form that catholyte solution.
[0052] One non-limiting example of the operation of the electrolytic cell 200
is
described as follows: Anolyte solution containing approximately 60-100% polar
solvent such asformamide, methyl formamide, dimethyl formamide, acetamide,
methyl acetamide, dimethyl acetamide, ethylene glycol, propylene glycol, 1,2-
ethanediol, 1,2-propanediol, propylene carbonate, ethylene carbonate, diethyl
carbonate, N-methyl pyrrolidone, tetraethylene glycol dimethyl ether
(tetralglyme),
acetonitrile, dimethyl sulfoxide, liquid ammonia, methyl amine or 1,3-Dimethy1-
3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU) or combinations of the above, and
0-
40% apolar solvent such as N,N-dimethylaniline (DMA) or quinoline, and 1% to
saturation, sodium polysulfide relative to the total solvent, is fed into the
anode
compartment 206. The electrodes are energized such that there is an electrical
potential between the anode 212 and the cathode 220 that is greater than the
decomposition voltage which ranges between about 1.8 V and about 2.5 V
depending on the composition. Concurrently, sodium ions pass through the
divider
into the cathode compartment 204, sodium ions are reduced to the metallic
state and
plate onto the cathode belt 220, and polysulfide is oxidized at the anode such
that
17
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
low polysulfide anions become high polysulfide anions and/or elemental sulfur
forms
at the anode. While sulfur is formed it is dissolved into the anolyte solvent
in entirety
or in part.
[0053] The sodium plated onto the belt is removed from the cell as the cathode
belt is advanced then subsequently the alkali metal 222 is removed from the
cathode
belt 220 by scraping or melting outside of the cell. The catholyte is
comprised of a
polar solvent such as tetraglyme and a salt to increase the ionic
conductivity. For
example, in this case sodium halide salt such as sodium chloride can be used
to
increase the ionic conductivity and the decomposition voltage of sodium
chloride is
much higher than the decomposition of sodium polysulfide. The electrolytic
cell 200
is operated at a temperature below the melting temperature of sodium. To
minimize
cell heating due to resistive losses, the anode and cathode may be spaced
relatively
close to the divider 208, within a few millimeters. Adjustments to cell
temperature
can be made using a heat exchanger on the flow of anolyte entering and exiting
the
cell through ports 216, 218.
[0054] The cell shown in Figure 2 has a general horizontal orientation but
could
also be configured in a generally vertical or other orientation.
[0055] In the case of the alkali metal being sodium, the following typical
reactions
may occur in the electrolytic cell 200:
At the Cathode:
Na + + e- ¨> Na
At the Anode:
Na2Sx ¨> Na + + e-+ 1A Na2S(2x)
Na2Sx ¨> Na + + e- + 1/2 Na2Sx + x/16S8
Where x ranges from 0 to about 8 but may be greater than 8..
18
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0056] As noted above, because the liquid that was added to the cell 200
previously had undergone a "heat treating" process, this cell 200 does not
have
organic materials foul the electrodes.
[0057] Referring now to Figure 3, a schematic diagram of a method 600 for
regenerating sulfur and an alkali metal from an oil source is described.
Specifically,
the method 600 includes an oil source 102 of the type described herein. This
oil
source 102 is reacted within a reactor 104 with a quantity of an alkali metal
106, in
the manner outlined above. Once reacted, a liquid material 602 is produced.
(This
liquid material 602 may simply be referred to as "liquid 602.") This liquid
602 may be
the upgraded oil product. In addition to liquid product 602, a quantity of
solid
materials 605 (which may be simply called "solids") are produced.
[0058] The produced solids 605 may be washed with a light petroleum substance
such as hexane, heptane, toluene, or mixtures of these substances, or natural
gas
condensate, or the like to remove adhered liquid product 602. The light
petroleum
substance may be stripped away by distillation, for example, to leave behind
liquid
product. This liquid product may then be re-added to the liquid 602. The light
petroleum substance, which was stripped away, may be re-used in washing
another
batch of solids 605.
[0059] The solids 605 may include quantities of heavy metals, coke, organic
solids, sodium sulfide, sodium nitride, etc. These solids 605 may then be
subjected
to a heat treatment step 610. In this heat treatment, the solids 605 are
heated in a
substantial absence of oxygen or water, for example under the presence of
nitrogen,
an inert gas or hydrocarbon gases such as methane. Such heating may involve
heating the solids 605 to a temperature above 400 C and preferably above 500
C.
As part of this heat treatment procedure 610, a quantity of treated solids 615
are
19
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
produced. Further, during the heat treatment procedure, a quantity of gases
612
(such as methane or other organic gases) is also produced. It is believed that
this
heat treatment step 610 operates to convert some of the organic products, such
as
coke, within the solids 605 into methane or other volatile organics, such that
these
gases are removed from the solids 605. As a result of the gases 612 being
emitted,
the weight of the treated solids 615 is generally less than the weight of the
solids 605
(given that some of the mass of the solids 605 has been lost as organic
gases.)
After undergoing this heat treatment procedure, the solids 615 may be more
granular
than they were previously.
[0060] Once treated by this heat treatment step 610, the treated solids 615
may
then be dissolved, in step 620, in a solution comprising a polar solvent 621.
Once
dissolved, the material is added to a separator 114. Within this separator
114, solids
630 will be removed. Such solids 630 may include residual coke and heavy metal
products. Such solids 630 can literally fall to be bottom of the separator
114, and
thus may be removed by gravimetric processes, filtration or other methods.
[0061] Once the solids 630 are removed, a resulting liquid 632 is formed. This
liquid 632 may be yellowish to clear in color as a result of the presence of
dissolved
sodium sulfide. (Polysulfide and/or hydrogen sulfide anions may also be
present.)
This liquid 632 may be introduced into an electrolytic cell 120. Any
electrolytic cell
may be used, including the cells 120, 200 described above. Other types of
electrolytic cells, including those described in the '270 patent or the '874
application,
may also be used. While in this cell 120, electricity is added to conduct an
electrolytic reaction which operates to oxidize the sulfide anions into
polysulfide ions
and sulfide and polysulfide ions into sulfur 128 (which may be collected, re-
used,
sold, etc.) as well as regenerated alkali metal 652. This regenerated alkali
metal 652
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
may then be re-used in the reaction vessel 104 as a means of upgrading a
further
batch of oil products. A portion of the anolyte from the cells 120 may serve
as the
polar solvent 621.
[0062] Most sodium is produced commercially from electrolysis of sodium
chloride
in molten salt rather than sodium polysulfide, but the decomposition voltage
and
energy requirement is about half for polysulfide compared to chloride as shown
in
Table 1.
Table 1. Decomposition voltage and energy (watt-hour/mole) of sodium and
lithium
chlorides and sulfides
NaCI Na2S LiCI Li2S
V 4.0 <2.1 4.2 2.3
Wh/mole 107 <56 114 60
[0063] The open circuit potential of a sodium/polysulfide cell is as low as
1.8V
when a lower polysulfide, Na2S3 is decomposed, while the voltage rises with
rising
sulfur content. Thus, it may be desirable to operate a portion of the
electrolysis
using anolyte with lower sulfur content. In one embodiment, a planar NaSICON
or
Lithium Titanium Phosphate (LTP) membrane is used to regenerate sodium or
lithium, respectively. NaSICON and LTP have good low temperature conductivity
as
shown in Table 2. The conductivity values for beta alumina were estimated from
the
300 C conductivity and activation energy reported by May. G. May, J. Power
Sources,3, 1 (1978).
21
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
Table 2. Conductivities of NaSICON, LTP, Beta alumina at 25 C, 120 C
Conductivity mS/cm
Beta alumina
Temperature C NaSICON LTP (est)
25 0.9 0.9 0.7
120 6.2 1.5 7.9
[0064] The anolyte solution may be preferably selected to dissolve
polysulfides
and sulfur. U.S. Patent No. 6,852,450 to Hwang et al. discloses a high cathode
(sulfur electrode) utilization by using a mixture of polar and apolar
solvents. The
polar solvents were useful for dissolving most of the polysulfides that are
polar in
nature and the apolar solvent is useful for dissolving the sulfur that is
apolar in
nature. A mixture of polar and apolar solvents may be used in anolyte solution
within
the scope of the present invention, but it is not required. If the
electrolytic cells are
operated above the melting temperature of sulfur, it may not be necessary to
use an
apolar solvent for the purposes of completely dissolving the sulfur, but the
apolar
solvent will likely reduce the polarization of the anode. Hwang measured the
solubility of sulfur and found numerous solvents with relatively high
solubility. Hwang
did not report the solubility of polysulfides. The top eight solvents were
cyclohexane,
benzene, trifluortoluene, toluene, fluorbenzene, tetrahydrofurane (THF) and 2-
methyl
tetrahydrofurane (2-MeTHF). The first six have solubilities above 80 mM while
the
last two have solubilities above 40 mM. To separate the sulfur, a portion of
the
anolyte from the high polysulfide cells will be bled off and processed, as
discussed
herein. Some of the sulfur may be removed by cooling and gravimetrically
22
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
separating or through filtration. Other methods may also be used such as
vaporizating the apolar solvent then using gravimetric or filtration means.
[0065] Table 3 lists the eight solvents with highest sulfur solubility based
on the
findings of U.S. Patent No. 6,852,450. This patent did not specify but the
solubilities
listed are probably for temperatures near 25 C and would be higher at
elevated
temperatures. Table 3 also lists the boiling points of those solvents. The
data is
arranged in order of boiling point temperature. Based on this data, the most
suitable
solvents to be added to the anolyte are xylene, toluene and trifluorotoluene.
Operation at pressures above ambient may be desirable to keep the solvent from
vaporizing at operating temperatures near 120 C, particularly since most of
the
domestic shale oil would be processed at elevations between 4000-8000 feet
above
sea level.
Table 3. Sulfur solubility and boiling point of eight solvents, high
solubility
Solvent Sulfur Boiling Point
Solubility (mM) ( C)
Xylene 77 140
Toluene 84 111
Trifluorotoluene 78 103
Fluorobenzene 83 85
Cyclohexane 93 81
Benzene 88 80
2-Me THF 44 80
THF 48 66
23
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0066] Conversely, Table 4 lists eight solvents with low sulfur solubility
based on
U.S. Patent No. 6,852,450. Composing anolyte from one or more solvents from
Table 3 and one or more solvents from Table 4 may be desirable such that
apolar
solvent dissolves sulfur and a polar solvent dissolves the polar polysulfide.
If the
process is run in stages, it may be useful to have the polar solvent in the
low
polysulfide cells because they should contain negligible amounts of sulfur.
Based on
boiling point in Table 4, tetraglyme, and diglyme would be the best candidate
solvents for the anolyte, given operating temperature of 120 C.
Table 4. Sulfur solubility and boiling point of eight solvents, low solubility
Solvent Sulfur Boiling Point
Solubility (mM) ( C)
Tetraglyme 1.4 275
Diglyme 1.5 162
Isopropanol 1.0 108
Ethyl Propianal 1.7 99
Dimethyl Carbonate 0.8 90
Dimethoxy ether 1.3 85
Ethanol 0.9 78
Ethyl acetate 1.5 77
[0067] Sulfur has been found to be soluble to an extent in tetraglyme and the
solubility rises with increasing temperature. Adding an apolar solvent such as
N,N-
dimethylaniline (DMA) increases the sulfur solubility. The sulfur solubilities
versus
temperature for tetraglyme, DMA and mixture of tetraglyme and DMA, 80:20 by
weight are shown in Table 5 below:
24
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
Table 5: Sulfur solubility in solvents versus temperature (wt%)
Temp C TG DMA 80:20 TG:DMA
25 0.16 3.37 0.46
50 1.01 6.92 1.26
70 1.16 10.7 1.89
[0068] Tetraglyme alone can dissolve sulfur formed at the anode to an extent,
particularly if the cells operate at elevated temperatures above 50 C.
Addition of
selected solvents such as DMA enables the solvent to dissolve more sulfur,
preventing polarization at the anode.
[0069] If the electrolytic cells operate at an even slightly elevated
temperature of
about 70 C, a stream of anolyte solution near saturation can be brought
outside the
electrolytic cell and chilled using a heat exchanger or other means to cause
sulfur to
precipitate. The sulfur can be removed by one of several means such as
filtration,
gravimetrically, centrifugation, and such. Sulfur has nearly two (2) times the
specific
gravity of the solvent mixture and is easily separated. The sulfur depleted
solvent
then can be returned to the anolyte to reduce the overall sulfur concentration
in the
anolyte.
[0070] Once the solution of sodium and sulfides are added to the cell, sulfur
may
be obtained. Figure 4 discloses a schematic of an exemplary embodiment of a
system 300 to remove sulfur from the anolyte solution. Referring to Figure 4,
warm
sulfur laden anolyte solution 302 enters heat exchanger 304. Coolant 306 from
a
chiller or cooling tower (not shown) cools down the anolyte through heat
exchange.
Coolant from the heat exchanger 308 returns back to the chiller. As the sulfur
laden
anolyte solution 302 is cooled, sulfur precipitates or forms a second liquid
phase as
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
the solubilty within the anolyte decreases. The chilled anolyte 310 enters an
enclosed thickener 312 to allow settling of solid phase sulfur or sulfur
liquid phase. A
stream heavily containing sulfur solids 314 flows to a rotary filter 316.
Liquid anolyte
flows into the filter while solid sulfur remains on the filter media on the
outside of the
drum 318. Overflow anolyte from the thickener 320 enters a tank 322 that also
receives make-up solvent mixture 324. Together this stream is used as a spray
326
to wash the sulfur filter cake. The sulfur filter cake is removed from the
rotary filter
enclosure by a conveyor means (not shown). Chilled and low sulfur bearing
anolyte
327 is pumped from the filter drum back to the electrolytic cell. The stream
326 may
be heat exchanged with stream 302 in a heat exchanger (not shown) to heat up
the
anolyte before returning it to the electrolytic cell and to reduce the
temperature of the
anolyte entering the chilled heat exchanger 304. Sulfur liquid phase may be
separated directly from the bottom of the thickener 312. It will be
appreciated that
many alternative approaches and variations to this process of removing sulfur
from
the anolyte solution are possible. It may also be appreciated that a second
phase of
liquid sulfur may form within the cells 120 and may settle in a thickener 312,
without
chilling.
[0071] Other anolyte solvents which may be utilized to increase sulfur
solubility in
the anolyte solution include: tetrahydrofuran, 2-methyl tetrahydrofuran,
benzene,
cyclohexane, fluorobenzene, thrifluorobenzene, toluene and xylene. Other polar
solvents which may be used to dissolve polysulfides include: tetraglyme,
diglyme,
isopropanol, ethyl propional, dimethyl carbonate, dimethoxy ether, ethanol and
ethyl
acetate, propylene carbonate, ethylene carbonate, diethyl carbonate and such.
[0072] Another non-limiting example on a process within the scope of the
present
invention is like the one disclosed above except lithium polysulfide is
decomposed.
26
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
Lithium ions pass through the divider and lithium metal is reduced at the
cathode
inside the cell and scraped off outside the cell.
[0073] The '270 patent discloses an embodiment in which after the oil stream
has
been reacted with an alkali metal, the product stream may further be reacted
with
H2S, thereby converting the sodium sulfide products into NaHS (and the nitride
products into ammonia gas and NaHS). It should be noted that once these
reactions
have occurred, the solid products (which contain the heavy metals and the NaHS
products) may be washed with the toluene (or other solvent) in the manner
outlined
herein. This washing liquid will then be removed and the formed liquid (which
includes upgraded oil products) may be re-added to the liquid upgraded oil
feedstock.
[0074] The washed solids may then be heat-treated, in the manner outlined
above. This heat treatment of the solids (which include NaHS and heavy metals)
occurs at a temperature above 400 or 500 C, and occurs under nitrogen,
methane,
or another non-oxidizing environment. During this heat-treating, some of the
organic
materials that were present in the solids (such as coke materials) will be
converted
into methane and removed from the solid. Thus, the mass of the solid materials
after
heat-treating may be less than the mass of the solids before heat-treating.
[0075] This heat-treated solid material (which contains NaHS) may then be
dissolved in a polar solvent so that the heavy metals may be separated out.
The
resulting liquid material, which includes dissolved NaHS and the polar
solvent, is
added to a cell so that the S and the Na may be recovered, in the manner
outlined
herein.
[0076] Referring now to Figure 5, another embodiment of a process 700 for
upgrading an oil feedstock is shown schematically. As will be described in
greater
27
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
detail herein, this process 700 removes sulfur, nitrogen and heavy metals from
an oil
feedstock 701, while at the same time, regenerates the alkali metal 702.
Specifically, the process 700 involves reacting an oil feedstock 701 with a
quantity of
an alkali metal 702. This reaction may occur within a reaction vessel 104 or
another
suitable vessel. This reaction produces a quantity of solid materials 705
(which may
also be referred to as "solids") as well as liquid materials 703 (which may
also be
referred to as "liquids"). The liquids 703 may be the upgraded oil stream that
has a
reduced amount of sulfur, nitrogen and heavy metals contained therein.
Additionally
and/or optionally, a gas 707 may be added to the reactor 104 to facilitate the
reaction
of the oil feedstock 701 and the alkali metal 702. This reaction with the gas
707
(which may be hydrogen, methane, or another hydrocarbon gas) is described
above
and in the '874 application.
[0077] As shown in Figure 5, the solids 705 and liquids 703 may be
separated
from each other. This separation may occur within a separator 706. This
separation
produces separated liquid materials 712 and separated solids 715. The
separated
solid materials 715 may then be washed, as shown in washing step 720. This
washing may involve washing 720 the solids 715 with an organic washing liquid
730
such as hexane, heptanes, toluene or mixtures of these substances, or natural
gas
condensate, or another hydrocarbon liquid. The purpose of this washing 720 is
to
collect any residual oil materials that may have been adhered to the solids
715.
(Once washed, the solids may be referred to as "washed solids" or washed solid
materials 725.)
[0078] After being used to wash the solids, the organic washing liquid 730 may
be
removed 735. More specifically, the washing liquid 730 will be evaporated off,
leaving the organic products that were adhered to the solids 715. These
resulting
28
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
products may then be added/re-mixed with the separated liquids 712, as shown
by
arrow 719.
[0079] The washed solids 725 may then be subjected to a heat treating step
744.
In this heat treating step, the solid materials 725 are heated a temperature
above
400 C (and more preferable to a temperature above 500 C). This heat treating
744
may occur in an atmosphere that has low oxygen and water content. In some
embodiments, this may involve heating the solids 725 in an atmosphere
comprising
one or more of the following gases: nitrogen, helium, neon, argon, krypton,
xenon,
radon, methane or another hydrocarbon or mixtures of the foregoing. It should
be
noted that the heat treating step 744 may cause the solid materials 725 to
lose
mass. This loss of mass also corresponds with an increase in the carbon to
hydrogen ratio of the solid material. In other words, the heat treating 744
converts
some of the coke/organic product within the solids 725 into gases 751 that are
emitted during the heat treatment but may be collected for gas products or
process
value. These gases may be methane or another hydrocarbon gas. (It is the loss
of
this gas 751 that causes the mass of the solids 725 to be reduced.) Moreover,
because a hydrocarbon gas is emitted (such as methane) the overall carbon to
hydrogen ratio within the solid materials 725 may be increased.
[0080]After the heat treating 744 has occurred, the remaining solid materials
753 (as
represented by an arrow), may be added to a solution comprising a polar
solvent
756. More specifically, the solid materials 753 are dissolved 752 (or
partially
dissolved) in a solution comprising a polar solvent 756. This solution
comprising a
polar solvent 756 has a boiling temperature above 130 C and specific gravity
less
than 2 g/cc. In some embodiments, the solution comprising a polar solvent 756
comprises one or more solvents selected from group consisting of: formamide,
29
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
methyl formamide, dimethyl formamide, acetamide, methyl acetamide, dimethyl
acetamide, ethylene glycol, propylene glycol, 1,2-ethanediol, 1,2-propanediol,
propylene carbonate, ethylene carbonate, diethyl carbonate, N-methyl
pyrrolidone,
tetraethylene glycol dimethyl ether (tetralglyme), acetonitrile, dimethyl
sulfoxide,
liquid ammonia, methyl amine methyl formamide, 1,3-Dimethy1-3,4,5,6-tetrahydro-
2(1H)-pyrimidinone (DMPU), and combinations thereof.
[0081] The solid materials 753 contain some sulfide, hydrogen sulfide and/or
polysulfide anions contained therein. Accordingly, the polar solvent 756 must
be
selected so that at least some sulfide, hydrogen sulfide and/or polysulfide
anions
dissolve 752 therein.
[0082] After the solid materials 753 are added to the polar solvent 756, a
separation 760 may occur in which any remaining solid materials 762 are
removed
from the polar solvent 756. As noted above, the polar solvent 756 will
dissolve or
partially dissolve sulfide, hydrogen sulfide or polysulfide anions;
accordingly, the
resulting liquid 770 may have a yellowish tint from the dissolved sulfur
moieties but
may also be clear. However, some solids may not dissolve in the solution
comprising polar solvent 756. Thus these solids, which are called remaining
solid
materials 762, can be removed from the liquid.
[0083] The solution comprising polar solvent 756 (which includes the liquid
770)
is added to an electrolytic cell 775. More specifically, the solution
comprising polar
solvent 756 may be added to an electrolytic cell 775 that includes an anolyte
compartment 780 and a catholyte compartment 784. The solution comprising polar
solvent 756 may be added to the anolyte compartment 780. The anolyte
compartment 780 may, at least partially, house an anode 791. The anolyte
compartment 780 also includes an anolyte 788. The solution comprising polar
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
solvent 756 mixes with/becomes part of the anolyte 788. The anolyte 788 is
preferably a liquid material. Further, a portion of the anolyte 788 may serve
as the
solution comprising polar solvent 756.
[0084] The catholyte compartment 784 at least partially houses a cathode 793.
The catholyte compartment 784 also includes a catholyte 787. The cell 775
further
comprises an alkali ion conductive membrane 795. This membrane 795 is
substantially impermeable to sulfide, hydrogen sulfide or polysulfide anions,
the
catholyte, the anolyte, and sulfur. This membrane 795 separates the catholyte
compartment 784 from the anolyte compartment 780. The alkali ion conductive
membrane 795 allows alkali metal ions to pass through the alkali metal ion
conductive membrane 795 from the anolyte compartment 780 to the catholyte
compartment 784. In some embodiments, the alkali ion conductive membrane 795
is selected from the group consisting of an alkali metal conductive ceramic, a
glass
ceramic; and a solid MSICON (Metal Super Ion CONducting) material, where M is
Na or Li.
[0085] During operation of the cell, an electrolytic reaction will occur. More
specifically, during operation, the electrolytic cell 775 may produce alkali
metal 798
in the catholyte compartment 784 (and thus regenerate the alkali metal 702).
Likewise, the electrolytic cell 795 (in the anolyte compartment 780) may
produce
elemental sulfur 797. More specifically, during operation of the electrolytic
cell 775,
sulfur moeities in the anolyte compartment 780 (from the polysulfide, sulfide
and/or
hydrogen sulfide anions) are reacted to form polysulfide ions and elemental
sulfur
797. Alkali metal ions in the catholyte compartment 784 are reacted to form
elemental alkali metal 798. In some embodiments, the cell 775 and/or the
anolyte
31
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
compartment 780 is maintained at a temperature that is greater than or equal
to 115
C such that the produced sulfur 797 is in the liquid phase.
[0086] In some embodiments, this operation of the electrolytic cell 775 may
occur
at a temperature that is below the melting temperature of the alkali metal
798. In
these embodiments, solid elemental alkali metal is produced (and may be, for
example, plated onto the cathode in the manner outlined above in connection
with
Figure 2). In other embodiments, the cell 775 or the catholyte compartment 784
may
be heated to a temperature above the melting point of the alkali metal 798
such that
the produced alkali metal is molten. If molten, the alkali metal may be
removed from
the catholyte compartment 784 in a variety of ways, gravimetric,
electromagnetic
pumping and other methods know by those skilled in the art handling molten
metals.
[0087] In addition to producing sulfur and alkali metal, polysulfides may be
produced.
[0088] In order to produce the alkali metal 798, the catholyte 787 in the
catholyte
compartment may comprise an alkali metal salt selected from the group
consisting of
an alkali metal chloride, bromide, iodide, perchlorate, and
hexafluorophosphate.
Further, the catholyte 787 may also include a catholyte solvent selected from
group
consisting of tetraglyme, diglyme, dimethyl carbonate, dimethoxy ether,
propylene
carbonate, ethylene carbonate, and diethyl carbonate. Also if the temperature
is
above the melting temperature of the alkali metal, the molten alkali metal may
serve
as the catholyte.
[0089] Referring now to Figure 6, a further process 800 is illustrated in
schematic
form. The process 800 relates to a method of upgrading an oil feedstock.
Specifically, in the process 800 a solid material is obtained 808. This solid
material
has been formed from the reaction of an oil feedstock with a quantity of an
alkali
32
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
metal, in the manner outlined herein. This solid material will then be heat
treated
812 in the manner outlined herein. Such heat treating 812 may involve heating
the
solid materials to a temperature above 400 or 500 C under a nitrogen
atmosphere
(or other inert atmosphere). This heat treating may cause the solid materials
to lose
mass as a result of some of the organic matter in the solid materials being
converted
into methane or other gases. The heat treated solid materials are represented
in
Figure 6 by arrow 814.
[0090]These heat treated solid materials 814 may then be dissolved 816 in a
solution comprising polar solvent 813. This dissolving forms a liquid material
832
and a solid material 830. As shown by Figure 6, the liquid materials may be
separated (using separating techniques 826) such that the liquid materials 832
are
isolated from the remaining solids 830. These remaining solids 830 may
comprise
heavy metals or other materials that were formed during the reaction between
the
organic oil feedstock and the alkali metal. It should be noted that the
solution
comprising polar solvent 813 used to dissolve the materials may have a boiling
temperature above 130 C and specific gravity less than 2 g/cc. This solution
comprising polar solvent 813 should be selected such that sulfide anions,
polysulfide
anions and/or hydrogen sulfide anions have at least some solubility in the
solution
comprising polar solvent 813.
[0091] As shown in Figure 6, the liquid materials 832, which includes the
solution
comprising polar solvent 813 and sulfide anions, polysulfide anions and/or
hydrogen
sulfide anions is added (as shown by arrow 840) to an electrolytic cell 875.
This
electrolytic cell 875 may be electrolyzed. In general, this electrolyzing may
occur at
a temperature that is greater or equal to 115 C so that any sulfur formed in
the cell
875 is in its liquid phase.
33
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0092] The cell 875 comprises an anode 893 and a cathode 891. The anode 893
is at least partially housed in an anolyte compartment 884. The anolyte
compartment 884 will generally include a liquid anolyte 887. The liquids 832
mix
with the liquid anolyte 887.
[0093] The cell 875 may also include a catholyte compartment 880. The anolyte
compartment 884 and the catholyte compartment 880 are separated by an alkali
metal ion conductive membrane 895.
[0094] The liquid anolyte 887 may include a quantity of sulfide anions 854, a
quantity of polysulfide anions 856 and/or a quantity of hydrogen sulfide
anions 855.
An anolyte solvent 846 (which may or may not be the same as the polar solvent
813
used in the dissolving step 816) is also part of the liquid anolyte 887. As
part of the
reaction at the anode 893, alkali metal ions 842 are formed. These alkali
metal ions
842 may be transported across the alkali metal ion conductive membrane 895
from
the anolyte compartment 884 to the catholyte compartment 880.
[0095] The catholyte compartment 880 also includes a liquid catholyte 888.
This
catholyte 888 includes a catholyte solvent 847, which may or may not be the
same
as the solvent 846 in the anolyte compartment 884. The catholyte solvent 847
may
be selected from group consisting of tetraglyme, diglyme, dimethyl carbonate,
dimethoxy ether, propylene carbonate, ethylene carbonate, and diethyl
carbonate. The catholyte 888 may further include an alkali metal salt that is
dissolved into alkali metal ions 842 and anions 844. In some embodiment the
alkali
metal salt is selected from the group consisting of an alkali metal chloride,
bromide,
iodide, perchlorate, and hexafluorophosphate. As shown in Figure 6, such
alkali
metal salts may dissolve and separate into their corresponding ions within the
liquid
catholyte 888.
34
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[0096] The reaction that occurs at the anode 893 will now be described.
Specifically, during operation of the electrolytic cell 875, sulfur moieties
in the anolyte
compartment are reacted to form polysulfide ions 856 and elemental sulfur 870
according to the following reactions:
Na2Sx ¨> Na + + e-+ 1/2 Na2S(2x)
Na2S, ¨> Na + + e- + 1/2 Na2Sx + x/1658
Where x ranges from 0 to about 8 but may be greater.
[0097] As shown in Figure 6, some of the anolyte 887 may be removed (as shown
by arrow 866) from the bottom 867 of the electrolytic cell 875. The removed
anolyte
866 comprises a portion of the produced elemental sulfur 870. This elemental
sulfur
870 may then be separated from the anolyte 866 via a separator 862. Once
separated, the sulfur 870 may then be sold, used etc. Further, after the
sulfur 870
has been separated, the anolyte may be returned to the cell 875, as shown by
arrow
869. The sulfur free anolyte may also serve as the solution comprising polar
solvent,
813. Figure 4 shows one example of the way in which sulfur 870 may be
separated;
however, other embodiments for separating the sulfur 870 may also be used.
[0098] At the cathode 891, alkali metal ions 842 are reduced to form alkali
metal
898. The way in which this occurs, and the way in which the alkali metal 898
may be
separated from the cathode 891, will now be described. The cathode 891
includes
an inside portion 891a that is within the catholyte compartment 880 (and thus
in
contact with catholyte 888) and an outside portion 891b that is outside of the
catholyte compartment. More specifically, the cathode 891 comprises a metal
band
877 that follows the path of rollers 871. The rollers 871 facilitate the
transfer of the
outside portion 891b to within the cell 875 and facilitate the movement of the
inside
portion 891a outside of the cell 875. The inside portion 891a of the cathode
891 can
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
be transferred outside the catholyte compartment 880 and the outside portion
891b
can be transferred inside the catholyte compartment 880 without substantially
interrupting the operation of the electrolytic cell 875. This may occur by
having the
alkali metal 898 plate onto the inside portion 891a of the cathode 891 while
this
portion is inside the catholyte compartment 880 and then the plated metal 898
is
removed (via brushes, scrapers, etc.) from the outside portion 891b of the
cathode
891 while this portion is outside the catholyte compartment 880. Of course,
those
skilled in the art will appreciate that Figure 6 shows only one example of the
way in
which the formed alkali metal 898 may be collected. Other embodiments may also
be used.
[0099] In view of the foregoing, it will be appreciated that the disclosed
invention
includes one or more of the following advantages:
operating an electrolytic cell to process an alkali metal sulfide or
polysulfide at
temperatures below the melting temperature of the alkali metal;
operating an electrolytic cell continuously or semi-continuously to process an
alkali metal sulfide or polysulfide at temperatures below the melting
temperature of
the alkali metal;
removing an alkali metal continuously or semi-continuously in solid form from
the cell;
removing high alkali metal polysulfides and dissolved sulfur continuously or
semi-continuously from the electrolytic cell, thereby reducing polarization of
the
anode by sulfur;
separating sulfur continuously or semi-continuously from a stream containing
a mixture of solvent, sulfur, and alkali metal polysulfides such that the
solvent and
36
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
alkali metal polysulfides are substantially recovered such that they can be
returned
back to an electrolytic process;
providing an apparatus and method for regenerating hydrogen sulfide from
and alkali metal hydrosulfide; and
operating the electrolytic cells at low temperatures and pressures, so that
the
electrolytic cell materials of construction can include materials which would
not
tolerate elevated temperature.
[00100] An additional post treatment that may be used to reduce the alkali
metal
content in the petroleum product is to use electrostatic separators to remove
suspended alkali metal sulfides or other alkali metal salts such as napthanic
acid
salts. The equipment utilized may be equipment found typically such as offered
by
AMR Process Inc. of Leduc, Alberta. The process of removing alkali metal
species
may further be assisted with the addition of water to the petroleum product
and
desalting with such electrostatic equipment.
[00101] Referring now to Figure 7, a schematic drawing of a process 900 for
upgrading the oil feedstock that includes this post-treatment method is
illustrated.
Specifically, a quantity of reacted oil feedstock 902 is obtained. This oil
feedstock
has been reacted by having an oil feedstock react with an alkali metal (such
as, for
example, a molten alkali metal). (As described above, this reaction may or may
not
include an additional gas, such as hydrogen, methane, etc.) Thus, in the
process
900, the reacted oil feedstock may be the upgraded oil 111, the liquid that
was sent
to separator 114, the liquid 602, the liquid 703 or another reacted oil
feedstock
material.
37
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
[00102] In the process 900, the reacted feedstock 902 may be filtered 910,
such that filtered solids 911 are removed. This filtering may remove suspended
solids such as suspended alkali metal sulfides or other alkali metal salts
such as
napthanic acid salts. Once filtered, a solution comprising polar solvent 912
may be
added to the liquid (as shown by line 919). In some embodiments, the solution
comprising polar solvent 912 comprises water 912a. However, other polar
solvents
may be used as the solution comprising polar solvent 912. This solution
comprising
polar solvent 912 may include water mixed with another polar solvent. This
polar
solvent 912/water 912a is designed to dissolve alkali metal salts that are
present in
the liquid stream.
[00103] Once the polar solvent 912 and/or water 912a has been added, the
polar solvent 912/water 912a may be separated 920 from the liquid 925 (which
is
represented by an arrow). The dissolved alkali metals salts and/or napthanic
acid
salts will generally separate into the solution comprising polar solvent
912/water
912a. Accordingly, when this phase is removed, the amount of these materials
in
the liquid 925 will be decreased. In order to further aid in this separation
process, an
electrostatic separator 930 may be used. An exemplary electrostatic separator
is
available from AMR Process Inc. of Leduc, Alberta, and may involve a desalting
process that is used with the liquid 925. Once this separation process is
completed,
the upgraded oil will have a reduced amount of alkali metal containing
materials
found therein, and thus may be worth more and/or more easily refined/processed
into a fuel product.
38
CA 02863357 2019-07-30
WO 2013/116340 PCT/US2013/023850
EXAMPLES
[00104] The following example is provided below which discusses one specific
embodiment within the scope of the invention. This embodiment is exemplary in
nature and should not be construed to limit the scope of the invention in any
way.
[00105] Sodium was reacted with bitumen which originally contained 5% sulfur.
Solids were separated from the treated bitumen by centrifugation. The solids
where
rinsed with toluene. The toluene rinse was heated to strip off the toluene
which was
collected in a condenser. The remaining liquid was added back to the product
liquid
(e.g., the liquid portion of the product obtained from the reaction of bitumen
and
sodium). 97% of the sulfur had been removed from the liquid product (according
to
test results) and the API gravity of the liquid product increased from 8 to
19.
[00106] The solids that were washed with toluene contained over 50% carbon and
were inter-mixed with sodium sulfide. The solids were heated to 600 C for one
hour
under nitrogen and cooled. Following the treatment the solids were powdery. X-
ray
diffractometry indicated the mixture of solid materials contained considerable
sodium
sulfide.
[00107] A polar organic solvent was mixed with the heat treated solids. The
polar
solvent liquid went from clear to yellow indicating dissolution of the sodium
sulfide.
The liquid solution was filtered to remove any undissolvable solids and then
the
liquid was added to an electrolysis cell with NaSICON membrane. The cell was
operated at 130 C and constant current of 60 milliamps per centimeter squared
current density. The cell initially had an open circuit potential of 1.8 V
which later
steadily rose to 2.5 V at which time 95% of the sulfur had been
electrochemically
reduced to its elemental form.
39
[00108] An electrolytic flow cell may utilize a 1" diameter NaSICON membrane
with approximately 3.2 cm2 active area. The NaSICON is sealed to a scaffold
comprised of a non-conductive material that is also tolerant of the
environment. One
suitable scaffold material is alumina. Glass may be used as the seal material.
The
flow path of electrolytes will be through a gap between electrodes and the
membrane. The anode (sulfur electrode) may be comprised of graphite or
titanium
among other materials. The cathode may be either aluminum or stainless steel.
It is
within the scope of the invention to configure the flow cell with a bipolar
electrodes
design. Anolyte and catholyte solutions may each have a reservoir and pump.
The
anolyte reservoir may have an agitator. The entire system may preferably have
temperature control with a maximum temperature of 150 C and also be
configured
to be bathed in a dry cover gas. The system preferably may also have a power
supply capable of delivering to 5 VDC and up to 100 mA/cm2.
[00109] As much as possible, materials will be selected for construction that
are
corrosion resistant with the expected conditions. The flow cell will be
designed such
that the gap between electrodes and membrane can be varied.
[00110] While specific embodiments of the present invention have been
illustrated
and described, numerous modifications come to mind without significantly
departing
from the spirit of the invention, and the scope of protection is only limited
by the
scope of the accompanying claims.
CA 2863357 2018-01-10